What is Anti Markovnikov addition?
In an addition reaction of a generic electrophile HX to an alkene or alkyne, the hydrogen atom of HX becomes bonded to the carbon atom that had the least number of hydrogen atoms in the starting alkene or alkyne.
Alkenes belong to the group of unsaturated hydrocarbons, that is, one molecule of alkene contains at least one double bond. Due to the presence of pi electrons, they show addition reactions in which an electrophile attacks the carbon-carbon double bond to form the additional products.
Table Of Contents
- Anti Markovnikov Addition
- Mechanism of Anti Markovnikov addition
- Recommended Videos
- Frequently Asked Questions – FAQs
Anti Markovnikov Addition
When HBr is added to unsymmetrical alkenes in the presence of peroxide, 1-bromopropane is formed contrary to 2-bromopropane (according to Markovnikov’s rule). This reaction is better known as anti-Markovnikov addition or Kharash effect after the name of M. S. Kharash who first observed it. This reaction is also known as Kharash effect or peroxide effect.
Anti Markovnikov addition is also an example of addition reaction of alkenes which is an exception to the Markovnikov’s rule. It is one of the few reactions following free radical mechanism in organic chemistry in place of electrophilic addition as suggested by Markovnikov. This reaction is observed only with HBr, not with HCl or HI.
Mechanism of Anti Markovnikov addition
Anti Markovnikov addition reaction is found to follow a free radical mechanism. The peroxide compound involved helps in the generation of free radicals. A general mechanism of anti-Markovnikov addition reaction is discussed below:
- Generation of free radical through homolytic cleavage of peroxide compound.
- Attack of generated free radical on hydrogen halide to form halide radical through hemolysis
- Attack of generated halide radical on alkene molecule to form alkyl radical through hemolysis.
- Attack of a generated alkyl radical on hydrogen halide to form alkyl halide through homolytic cleavage of hydrogen halide bond.
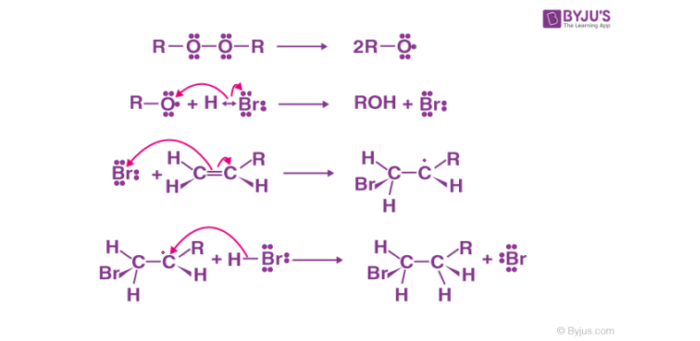
Fig: Anti Markovnikov addition mechanism
Recommended Videos
Anti-Markovnikov Addition Video Lesson

To learn more about Markovnikov addition, download BYJU’S the learning app.
Frequently Asked Questions – FAQs
What is anti Markovnikov rule simple definition?
The Anti-Markovnikov rule defines regiochemistry in which the substituent is attached to a less substituted carbon instead of the more substituted carbon. This is because substituted carbocation allows for more hyperconjugation and indution, resulting in a more stable carbocation.
How does Markovnikov rule work?
When a protic acid HX or other polar reagent is added to an asymmetric alkene, the acid hydrogen (H) group or electropositive component attaches to the carbon with more hydrogen substituents, while the halide (X) group or electronegative part attaches to the carbon with more alkyl substituents.
Why does anti addition occur?
Anti addition involves adding two substituents to opposite sides (or faces) of a double or triple bond, resulting in a drop in bond order and an increase in the number of substituents. Addition can have varying consequences on the molecule depending on the substrate double bond.
What is the peroxide effect or anti Markovnikov rule?
The peroxide effect, also known as anti-Markovnikov addition, occurs when HBr adds on the “wrong way around” in the presence of organic peroxides. Hydrogen bromide adds to propene via an electrophilic addition process in the absence of peroxides. As a result, the product anticipated by Markovnikov’s Rule is obtained.
Is bromination an addition reaction?
Any reaction or procedure that introduces bromine into a molecule. The electrophilic addition of Br2 to an alkene results in bromination. Electrophilic aromatic substitution brominates a benzene ring. A free radical substitution process is used to bromate a benzylic position.


in which compounds addition reaction will take place according to anti-markovnikov’s rule
Free radical addition reactions do not obey Markovnikov’s rule since the regioselectivity of the mechanisms of these reactions are not predicted by Markovnikov’s rule. These reactions are generally referred to as Anti-Markovnikov addition reactions.