What is Borax?
Borax is a compound consisting of an elementary substance called boron, united to oxygen and soda.
Borax formula, also known as sodium borate formula, sodium tetraborate formula or disodium tetraborate formula is explained in this article. Borax is a soft, colourless compound of Boron and can be dissolved in water. The main forms of borax are anhydrous and decahydrate salt and sometimes as a pentahydrate salt.
Table of Contents
- Borax Chemical Formula
- Recommended Videos/a>
- Natural Sources of Borax
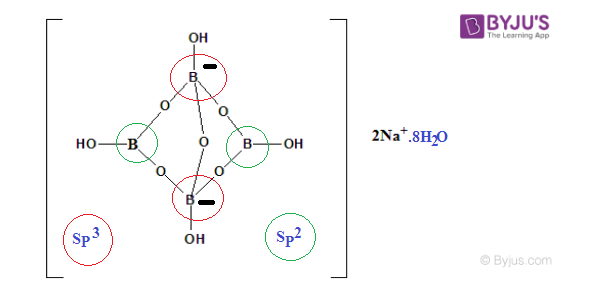
- Borax Structural Formula
- Borax Reaction
- Frequently Asked Questions – FAQs
Borax Chemical Formula
The most common form of borax is Borax decahydrate which has 4 Boron atoms, 17 oxygen atoms, 2 sodium atoms, and 20 hydrogen atoms. The Chemical and molecular formula of Borax is given as the following-
| Chemical Formula of Borax | Molecular Formula of Borax |
| Na2B4O7• 10H2O
or,
Na2B4O5(OH)4•8H2O |
H20B4Na2O17 |
Recommended Videos

Natural Sources of Borax
- Borax is naturally found in evaporite deposits which are produced by the recurrent evaporation of seasonal lakes.
- Naturally occurring borax is purified by recrystallization.
- Borax can also be synthesized from boron compounds.
Borax Structural Formula
Borax Reaction
Borax is mainly used in laundry and other cleaning products. It is also used to prepare boric acid by reacting with hydrochloric acid, i.e. HCl. This process was first used by Wilhelm Homberg. Apart from this, borax is also used in food, chemical, and pharmaceutical industries.
Borax reacts with hydrochloric acid to produce boric acid. The reaction is as follows:
Na2B4O7·10H2O + 2 HCl → 4 B(OH)3 + 2 NaCl + 5 H2O
Borax Reaction – Each of the sugar residues in the polymer has two hydroxyl groups positioned in the cis- form. This leads to an interesting and valuable reaction with dissociated borate ions that is characteristic of such polymers.
Frequently Asked Questions – FAQs
What is Borax chemistry?
A major boron compound, a mineral, and a salt of boric acid is borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate. Powdered borax, composed of soft, colourless crystals that readily dissolve in water, is white. Borax, sold commercially, is partly dehydrated.
Is Borax same as baking soda?
Baking soda and borax are derivatives of numerous chemical compounds widely found. Borax is categorized as a white solid sodium borate, whereas baking soda is categorized as white crystal sodium bicarbonate.
What is Borax used for?
The aim of Lewis structures is to provide a simple way for chemists to view molecules that allows accurate predictions about the actual molecules and structure and properties to be made.
Can Borax be used as a disinfectant?
Borax has many chemical characteristics that add to it’s cleaning ability. By converting certain water molecules to hydrogen peroxide (H2O2), Borax and other borates clean and disinfect. Borax helps this feature to clean and destroy unwanted pests.
Is borax and boric powder the same?
Two different formulations of the same compound are Borax and Boric Acid. Borax is a mineral used in cleaning materials that is extracted straight from the earth (a type of the element Boron). The extracted, distilled and refined type of boric acid is used in a number of chemical products.
Is Borax safe for humans?
Borax can not be swallowed safely. Borax is quick for the body to break down when it is either inhaled or swallowed, according to the Toxicology Data Network of the NLM. However, both severe poisoning and organ injury will result if inhalation or absorption occurs.
Why is borax dangerous?
When penetration happens by skin or eye touch, inhalation or swallowing, Borax can be irritating. Poison studies indicate that misuse of borax-based pesticides with symptoms such as vomiting, eye irritation , nausea, skin rash, oral irritation, and respiratory effects can result in acute toxicity.
To learn more such chemistry topics, keep visiting BYJU’S.
Read more:


Thanks