Let’s start by understanding what Coagulation is first. Well, according to the general definition, coagulation is one of the various properties exhibited by colloidal solutions. A colloid is a heterogeneous mixture of one substance of very fine particles (dispersed phase) dispersed into another substance (dispersion medium).
Table of contents
- Definition of coagulation of colloidal solution
- Coagulation techniques
- Hardy Schulze Law of coagulation
- Coagulation of lyophilic solutions
- Coagulation of lyophobic solutions
- FAQs
Definition of coagulation of colloidal solution
Coagulation is a process of aggregation or accumulation of colloidal particles to settle down as a precipitate.
Substances like metals, their sulfides etc. cannot be simply mixed with the dispersion medium to form a colloidal solution. Some special methods are used to make their colloidal solutions. Such kinds of sols are known as lyophobic sols. These kinds of colloidal solutions always carry some charge on them. Charge present on the colloidal sols determines their stability. If by any chance we can remove the charge present on the sol, the particles get closer to each other, and they accumulate to form aggregates and precipitate under the action of gravity. This process of accumulation and settling down of particles is further known as coagulation or precipitation.
Coagulation Techniques:
The process of coagulation can be carried out in the following ways:
- By electrophoresis: In this method, the colloidal particles are forced to move towards the oppositely charged particles, and then they are discharged and collected at the bottom.
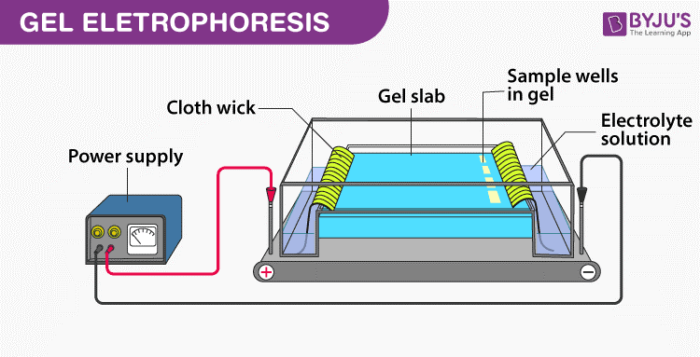
2. By mixing two oppositely charged sols: In this type of coagulation equal amounts of oppositely charged particles are mixed, they cancel out their charges and then precipitate.
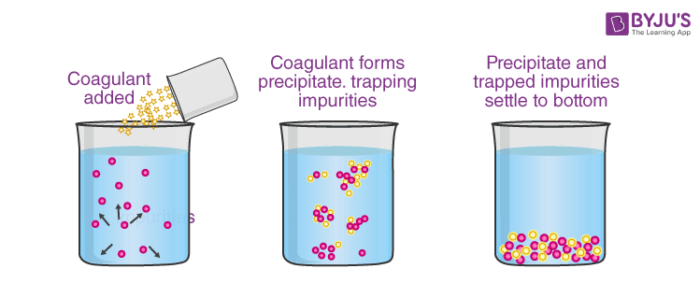
Fig. Coagulation by mixing two oppositely charged sols
3. By boiling: Whenever we boil a sol, the molecules of the dispersion medium start colliding with each other and with the surface, this, in turn, disturbs the adsorption layer. This reduces the charge on the sol due to which the particles settle down.
4. By persistent dialysis: Under the persistent dialysis parts of electrolytes are removed completely and the sol loses its stability and ultimately coagulates.
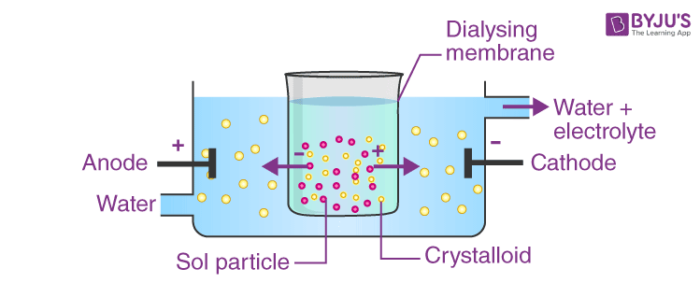
Fig. Coagulation by Persistent Dialysis
Hardy Schulze Law of coagulation:
According to Hardy Schulze law the quantity of electrolyte required to coagulate a definite amount of colloidal solution depends upon the valency of the coagulating ion.
- The coagulating ions are the ions of electrolyte which carry the opposite charge of the colloidal particles.
- The greater the valency of the coagulating ion, greater the coagulation power.
- For coagulation of a negatively charged sol (As2S3) the coagulating ion is cation, the power of coagulating value are given below :
| Electrolyte | Cation | Coagulating value |
| NaCl | Na+ | 52 |
| MgCl2 | Mg2+ | 0.72 |
| BaCl2 | Ba2+ | 0.69 |
| AlCl3 | Al3+ | 0.093 |
Coagulation ∝ 1/ Coagulating value (Coagulation is inversely proportional to coagulating value)
So the order of coagulating power of cations for negatively charged sol As2S3.
Al3+ > Ba2+ > Mg2+ > Na+
- For coagulation of a Positively charged sol (Fe(OH)3) the coagulating ion is Anion, the power of coagulating value are given below :
| Electrolyte | Anion | Coagulating value |
| KBr | Br – | 138 |
| K2SO4 | SO42- | 0.210 |
| K2C2O4 | C2O42- | 0.238 |
| K3[Fe(CN)6] | [Fe(CN)6]3- | 0.096 |
Coagulation ∝ 1/ Coagulating value
So the order of coagulating power of anion for Positively charged sol Fe(OH)3.
Br– < SO42- < [Fe(CN)6]3-
Coagulation of lyophilic solutions:
Stability of lyophilic sol depends on the following two factors
- Charge on the colloidal particles
- Solvation of the colloidal particles
When the above two factors are removed then only lyophilic sols can be coagulated. This can be done by either adding an electrolyte or a suitable solvent.
Coagulation of lyophobic solutions:
Lyophobic sols are less stable than lyophilic colloid. Hence they are more easily coagulated.
The stability of lyophobic sol is only due to charge on the colloidal particles. This factor can be removed by adding only electrolyte.
This was just a brief layout of coagulation of lyophilic and lyophobic colloids. To know more about the process of coagulation, you can register with BYJU’S and download our app.
Frequently Asked Questions- FAQs
What causes coagulation in a colloidal solution?
Charge present on the colloidal sols determines their stability. If we can remove the charge present on the sol, the particles get closer to each other, and they accumulate to form aggregates and precipitate under the action of gravity. So the electrolyte is added to the sol to neutralize the charge and settling down particles that causes coagulation or precipitation.
What is coagulation value?
The amount of electrolytes added to 1 liter of a colloidal solution to form coagulation or precipitation is called coagulation value.
What is the Hardy Schulze rule?
The quantity of electrolyte required to coagulate a definite amount of colloidal solution depends upon the valency of the coagulating ion is known as Hardy Schulze rule.
What modification can you suggest in the Hardy Schulze rule?
Hardy Schulze rule should be modified on the basis of polarizing power of the flocculating ion causing precipitation. Hence the modified law will be as: Greater the polarizing power of the flocculation ion, the greater will be its power to cause precipitation.
What is a coagulating ion?
The coagulating ions are the ions of electrolyte which carry the opposite charge of the colloidal particles.


Very good explanation for coagulation in chemistry
Thank you byjus