What is Galvanic Cell?
An electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy is known as a galvanic cell or a voltaic cell.
Galvanic cell Voltaic cell is an electrochemical cell that makes use of chemical reactions to generate electrical energy.
Table of Contents
- Principle of Galvanic (Voltaic) Cell
- Parts of Galvanic Cell
- Working of Galvanic Cell
- Example of Galvanic Cell
- Recommended Videos
- Frequently Asked Questions – FAQs
Let us understand how a voltaic or galvanic cell is created.
In oxidation-reduction reactions, electrons are moved from one species to another species. Energy is released if the reaction occurs spontaneously. Therefore, the released energy is used to do useful work. To tackle this energy, it is required to split the reaction into two separate half-reactions viz. oxidation and reduction. With the help of two different containers and wire, the reactions are put into them to drive the electrons from one end to the other end. This creates a voltaic cell.
Principle of Galvanic (Voltaic) Cell
Electric work done by a galvanic cell is mainly due to the Gibbs energy of spontaneous redox reaction in the voltaic cell. It generally consists of two half cells and a salt bridge. Each half cell further consists of a metallic electrode dipped into an electrolyte. These two half-cells are connected to a voltmeter and a switch externally with the help of metallic wires. In some cases, when both the electrodes are dipped in the same electrolyte, a salt bridge is not required.
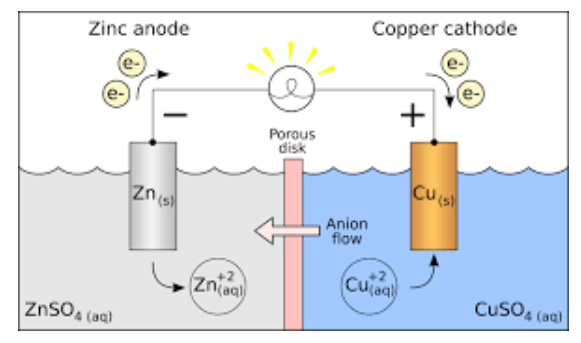
Galvanic Cell (Voltaic Cell) Diagram
Parts of Galvanic Cell
- Anode – Oxidation occurs at this electrode.
- Cathode – Reduction occurs at this electrode.
- Salt bridge – Contains electrolytes which are required to complete the circuit in a galvanic cell.
- Half-cells – reduction and oxidation reactions are separated into compartments.
- External circuit – Conducts the flow of electrons between electrodes
- Load – A part of the circuit utilizes the electron to flow to perform its function.
Working of Galvanic Cell
- In a galvanic cell, when an electrode is exposed to the electrolyte at the electrode-electrolyte interface, the atoms of the metal electrode have a tendency to generate ions in the electrolyte solution leaving behind the electrons at the electrode. Thus, making the metal electrode negatively charged.
- While at the same time metal ions in the electrolyte solution too, have a tendency to deposit on a metal electrode. Thus, making the electrode positively charged.
- Under equilibrium condition, charge separation is observed and depending on the tendencies of two opposing reactions, the electrode can be positively or negatively charged. Hence, a potential difference is developed between the electrode and electrolyte.
- This potential difference is known as electrode potential.
- Out of two electrodes, the electrode at which oxidation takes place is called anode while the electrode at which reduction takes place is called cathode.
- The anode has a negative potential with respect to the solution while the cathode has a positive potential with respect to the solution.
- Thus, a potential difference develops between two electrodes of the galvanic cell. This potential difference is known as cell potential.
- When no current is drawn from the galvanic cell, cell potential is known as the electromotive force of the galvanic cell.
- When the switch is set on, due to the potential difference, electrons flow from the negative electrode to the positive electrode.
Example of Galvanic Cell
Electrochemical or galvanic cells were introduced as a tool for studying the thermodynamic properties of fused salts more than a century ago. Daniel’s cell is an example of a galvanic cell that converts chemical energy into electrical energy. In Daniel’s cell, copper ions are reduced at the cathode while zinc is oxidized at the anode.
Reactions of Daniel cell at cathode and anode are:
At cathode: Cu 2+ + 2e– → Cu
Recommended Videos

Frequently Asked Questions – FAQs
What is the function of a galvanic cell?
The electrochemical cell type is a galvanic cell. It is used to supply electrical current through a redox reaction to the transfer of electrons. A galvanic cell is an example of how to use simple reactions between a few elements to harness energy.
How do you make a voltaic cell?
The basic cell or voltaic cell is made up of two electrodes, one of copper and the other of zinc dipped in a glass vessel solution of dilute sulfuric acid. The current flows from copper to zinc outside the cell and from zinc to copper inside the cell when the two electrodes are connected externally with a piece of wire.
Why cathode is positive in the galvanic cell?
The anode is the electrode where oxidation (loss of electrons) occurs; it is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. Therefore, the cathode is a neutral electrode; because there are reduced positive ions of metal atoms.
Is Daniel’s cell a galvanic cell?
Sometimes known as a voltaic cell or Daniell cell is a galvanic cell. One example of a galvanic cell is the common household battery. The electrons flow from one chemical reaction to another occurs through an external circuit that results in the current.
How do you represent a galvanic cell?
A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. The reactions to oxidation and reduction are divided into half-cell compartments.
For detailed discussions on different cells like a galvanic cell or voltaic cell, or about the Gibbs energy change spontaneity of a process visit BYJU’S.
Read more:


Explain electrolytic CEll
Electrolytic cells are electrochemical cells that facilitate non-spontaneous redox reactions with the help of an external electric current.