History of the Periodic Table
Earlier scientists assumed that the properties of elements are periodic functions of their atomic masses. On the basis of this assumption, Mendeleev placed 63 elements in a vertical column called groups and in horizontal rows called periods.
This method was rejected as it could not explain the position of certain elements, rare earth metals, and isotopes. A scientist named Henry Moseley removed these defects and put forward the modern periodic table with the modern periodic law.
Table of Content
Recommended Videos on Modern Periodic Table
Moseleys Periodic Law:
In the year 1913, Henry Moseley studied the frequencies of the X-rays Which were emitted when certain metals were bombarded with high speed electrons. He found that in all the cases, the square root of the frequency was directly proportional to the atomic number of the atom of the metals. These studies believe that atomic number is the fundamental property of an element. In the above observation Moseley gave the Modern Periodic Law which states that:
Physical and chemical properties of the elements are the periodic functions of their atomic numbers.
Modern Periodic Table:
A tabular arrangement of elements in groups and periods which highlights the regular trends in properties of elements is defined as the periodic table.
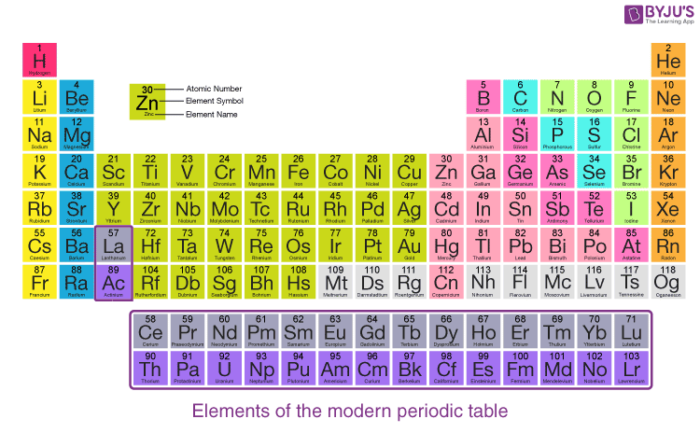
Features of Modern Periodic Table
There are eighteen vertical columns known as groups in the modern periodic table which are arranged from left to right and seven horizontal rows which are known as periods.
| Group number | Group name | Property |
| Group 1 or IA | Alkali metals | They form strong alkalis with water |
| Group 2 or IIA | Alkaline earth metals | They also form alkalis but weaker than group 1 elements |
| Group 13 or IIIA | Boron family | Boron is the first member of this family |
| Group 14 or IVA | Carbon family | Carbon is the first member of this property |
| Group 15 or VA | Nitrogen family | This group has non-metals and metalloids |
| Group 16 or VIA | Oxygen family | They are also known as chalcogens |
| Group 17 or VIIA | Halogen family | The elements of this group form salts. |
| Group 18 | Zero group | They are noble gases and under normal conditions they are inert. |
Also, Read:
| History Of Modern Periodic Table | Trends Of Chemical Reactivity in Periodic Table |
Classifications Of Elements in the Periodic Table
The elements of group 1, 2, 13, 14, 15, 16, and 17 are known as the main group elements or normal elements. The elements of groups 3, 4, 5, 6, 7, 8, 9, 11 and 12 are known as the transition elements. Group 18 is called noble gases or inert gases. Their outermost shell is completely filled. Due to this stable electronic configuration, they generally don’t react with the other elements.
When we talk about the periods of a modern periodic table, one should keep in mind that the number of shells present in an atom determines its period number. The elements of period one will have only one shell, elements of period two will have two shells and so on. The first period of the modern periodic table is the shortest period as it contains only two elements. The period number two and three consists of eight elements each and is known as short groups. Period four and five have eighteen elements and are known as the long group. In the modern periodic table, group number 3 of period six contains the lanthanide series which are the rare earth elements. We have radioactive elements (actinides) present in group 3 of period seven.
So we have seen the properties of the modern periodic table. To learn more about the periodic table and other concepts of chemistry, register with BYJU’S.
Frequently Asked Questions – FAQs
Which elements belong to group 15 of the modern periodic table?
In the modern periodic table of elements, the following fall under group 15:
- Nitrogen (N)
- Phosphorus (P)
- Arsenic (As)
- Antimony (Sb)
- Bismuth (Bi)
These elements are collectively referred to as the pnictogens (or the nitrogen family).
What trends in electronegativity can be seen in the modern periodic table of elements?
How are the elements classified into different blocks in the modern periodic table?
What are the f-block elements?
The f-block elements are the elements of the modern periodic table whose valence electrons lie in f-orbitals. These elements can be broadly classified into two categories.
- The lanthanides – elements whose valence electrons lie in the 4f orbital.
- The actinides – elements whose valence electrons lie in the 5f orbital.


Tq it’s helpful for me
Good