What is Nylon?
Nylon is the most useful synthetic material with applications varying from daily life activities to industries. It is a plastic which can be drawn into fibres or moulded into daily products for making amenities. We can live our entire life with nylon on our side. You hop across the nylon carpet to the kitchen, eat your breakfast on a nylon bowl after cleaning your teeth with a toothbrush whose bristles are made of nylon. A nylon umbrella over your head is used to move out of the house in heavy sunlight or to keep out of the rain.
The Science of Nylon
The term nylon points towards a polymer family known as linear polyamides. There are two approaches to making nylon for fibre applications. In the first approach, the molecules that consist of an acidic group (COOH) on every end react with molecules that contain amino (NH2) groups at each end. The resulting nylon gets a name based on the number of carbon atoms that separate two amines and two acidic groups. Hence, nylon 6,6 is widely used as fibres made from adipic acid and hexamethylenediamine.
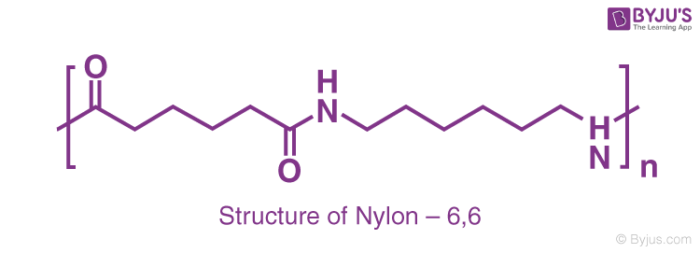
The salt which is formed by two compounds is known as nylon that has an exact ratio of 1:1 acid to base. This salt is dried and then heated under vacuum to remove water and form the polymer.
In the other approach, a compound that contains an amine at one end and acid at the other are polymerized to produce a chain with repeating units of (-NH-[CH2]n-CO-)x. The nylon is referred to as nylon 6 if n = 5 which is another common form of this polymer. The commercial production of nylon 6 starts with caprolactam that use an open-ring polymerization.
In both the approaches, the polyamide is melt and drawn after cooling to obtain the desired properties of every intended use.
Properties of Nylon
- Lustrous
- Elastic
- Very strong
- Damage resistant to oil and many chemicals
- Resilient
- Does not absorb water
- Dries quickly
Types of Nylon
Nylon 6 – It was developed by Paul Schlack. It is formed by ring-opening polymerization.
Nylon 510 – It is obtained from sebacic and pentamethylene diamine acid.
Nylon 1,6 – It is produced from dinitriles with the help of acid catalysis.
Nylon 66 – Wallace Carothers patented nylon 66 with the use of amide.
Uses of Nylon
- Clothing – Shirts, Foundation garments, lingerie, raincoats, underwear, swimwear and cycle wear.
- Industrial uses – Conveyer and seat belts, parachutes, airbags, nets and ropes, tarpaulins, thread, and tents.
- It is used to make a fishnet.
- It is used as plastic in manufacturing machine parts
Learn more about Nylon and other polymers and their uses on our app – BYJU’S The Learning App.
Frequently asked questions
Nylon is commonly used for what purpose?
To manufacture plastic machine parts because it is cost-effective and long-lasting.
Mention one advantage of Nylon.
It has high insulation and resistance to corrosion.
How nylon is made?
Appropriate monomers are combined to form a long chain through a process of the condensation polymerization reaction.
Read more:


My friend Tomas wanted to post a comment but was too scared, here’s his comment. “Awesome.
Good and excellent
What is use of nylone
Nylon is used for a variety of applications, including clothing, reinforcement in rubber material like car tires, for use as a rope or thread, and for many injection molded parts for vehicles and mechanical equipment. Click here to learn more about the uses and properties of Nylon.