What is Perchloric acid?
HClO4 is a chlorine oxoacid with chemical name Perchloric acid. It is also called Hyperchloric acid (HClO4) or hydroxidotrioxidochlorine. Between 50% -72% acid is a clear odourless colourless aqueous solution. It is corrosive to tissue and metals. When closed containers are exposed to heat for a long duration can rupture violently.
Properties of Perchloric acid – HClO4
| HClO4 | Perchloric acid |
| Molecular weight of HClO4 | 100.46 g/mol |
| Density of Perchloric acid | 1.768 g/cm3 |
| Melting point of Perchloric acid | −17 °C |
| Boiling point of Perchloric acid | 203 °C |
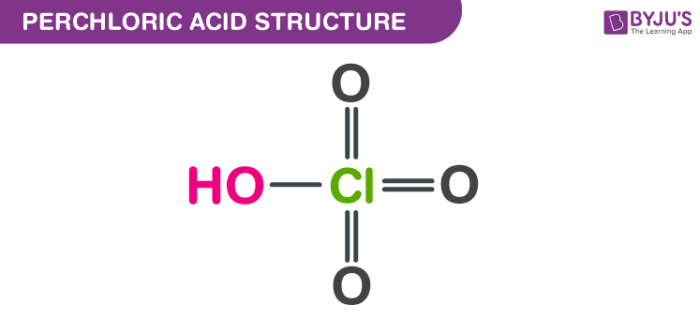
Perchloric acid structure – HClO4
HClO4 Uses (Perchloric acid)
- Perchloric acid is used as an oxidizer in the separation of sodium and potassium.
- Used in making explosives.
- Used for plating of metals.
- Used as a reagent to determine the 1H-Benzotriazole
- Used as a catalyst.
- Used in rocket fuel.
- Used for electropolishing or etching of molybdenum.
Production of Perchloric acid
At an industrial level it can be produced in two methods. In the traditional method makes full use of the high aqueous solubility of sodium perchlorate (NaClO4). Treating this solution with hydrochloric acid (HCl) produces perchloric acid by precipitating solid sodium chloride. The reaction is as follows:
NaClO4 + HCl → NaCl + HClO4
The other method is more direct and keeps away from salts and requires anodic oxidation chlorine solution at a platinum electrode.
It can also be prepared in laboratories by treating barium perchlorate (Ba(ClO4)2) with sulphuric acid (H2SO4) which precipitates barium sulfate (BaSO4) and leaves perchloric acid. Alternately it can be prepared by mixing nitric acid (HNO3) with ammonium perchlorate (NH4ClO4) and add hydrochloric acid while its boiling.
Health hazards
Inhaling vapours of this compound causes burning sensation in the throat and nose, irritation in lung along with coughing. Prolonged exposure causes vomiting. Ingesting this compound can cause blistering and burns in the stomach. When heated it liberates corrosive, irritating, and toxic gases.
Frequently Asked Questions
What are the uses of perchloric acid?
The primary application of perchloric acid is its use as a precursor to ammonium perchlorate, which is an inorganic compound which is a vital component of rocket fuel. Therefore, perchloric acid is considered to be a very important chemical compound in the space industry. This compound is also used in the etching of liquid crystal display systems (often abbreviated to LCD). Therefore, perchloric acid is widely used in the electronics industry as well. This compound is also used in analytical chemistry owing to its unique properties. Perchloric acid also has several important applications in the extraction of materials from their ores. Furthermore, this compound is also used in the etching of chrome. Since it acts as a super acid, perchloric acid is considered to be one of the strongest Bronsted-Lowry acids.
How is perchloric acid prepared?
The industrial production of perchloric acid usually follows one of two different routes. The first route, often referred to as the traditional route, is a method of preparing perchloric acid that exploits the extremely high solubility of sodium perchlorate in water. The solubility of sodium perchlorate in water corresponds to 2090 grams per litre at room temperatures. Treatment of such a solution of sodium perchlorate in water with hydrochloric acid results in the formation of perchloric acid along with a precipitate of sodium chloride. This concentrated acid can, furthermore, be purified via the process of distillation. The second route involves the use of electrodes in which the anodic oxidation of chlorine which is dissolved in water takes place at a platinum electrode. However, the alternate method is considered to be more expensive.
Is perchloric acid dangerous?
Perchloric acid is an extremely powerful oxidant. Owing to its strong oxidizing properties, this compound exhibits very high reactivities towards most metals. Furthermore, this compound is highly reactive towards organic matter as well. This compound can be corrosive towards the skin. Therefore, adequate safety measures must be taken during the handling of this compound.
Learn more about the Structure, physical and chemical properties of HClO4 from the experts at BYJU’S.


Comments