What is Potassium Chlorate?
Potassium Chlorate is an inorganic compound with chemical formula KClO3.
It is also known as Fekabit or Kaliumchlorat. It is very flammable when mixed with combustible materials. It is a compound containing potassium, oxygen, and chlorine. It appears as a white crystalline substance in its pure form. It is the most widely used chlorate industry.
The aqueous solution of potassium chlorate is a colourless liquid that is denser than water. It could be toxic when ingested. When it comes in contact it can irritate your eyes, skin, mucous membranes. It has a cooling and saline taste.
Table of Contents
- Potassium Chlorate Structure
- Preparation of Potassium Chlorate
- Properties of Potassium Chlorate
- Uses Of Potassium Chlorate
- Frequently Asked Questions – FAQs
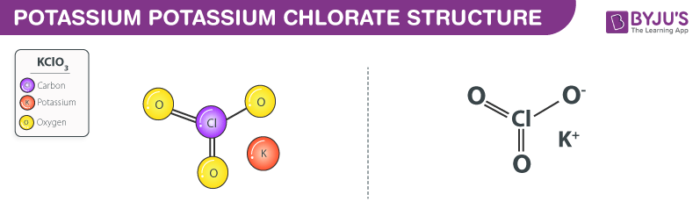
Potassium Chlorate Structure – KClO3
Preparation of Potassium Chlorate
Potassium chlorate can be produced in three ways:
1. On the industrial scale, it can be produced via the Liebig process:
Step 1. Consider hot calcium hydroxide.
Step 2. Pass chlorine into it.
Step 3. Add potassium chloride.
6 Ca(OH)2 + 6 Cl2 → Ca(ClO3)2 + 5 CaCl2 + 6 H2O
Ca(ClO3)2 + 2 KCl → 2 KClO3 + CaCl2
2. Potassium chlorate produced by disproportionation:
Step 1. Consider a sodium hypochlorite solution
Step 2. Metathesis reaction along with potassium chloride
3 NaOCl(aq) → 2 NaCl(s) + NaClO3(aq)
KCl(aq) + NaClO3(aq) → NaCl(aq) + KClO3(s)
3. Method three:
Step 1. Consider a hot solution of caustic potash
Step 2. Pass chlorine gas into it
3 Cl2(g) + 6 KOH(aq) → KClO3(aq) + 5 KCl(aq) + 3 H2O(l)
Properties of Potassium Chlorate – KClO3
| KClO3 | Potassium Chlorate |
| Molecular Weight/ Molar Mass | 122.55 g/mol |
| Density | 2.34 g/cm³ |
| Boiling Point | 400°C |
| Melting Point | 356°C |
Uses Of Potassium Chlorate (KClO3)
- Potassium chlorate along with silver fulminate is used in noise-makers such as snappers and crackers.
- It is used as an oxidizer in smoke grenades.
- It is used to generate oxygen gas in college and school labs.
- It is used in oxygen candles or chlorate candles.
- It is used in limelights to supply oxygen.
- It is used as a pesticide.
- It is used in growling gummy bears.
- It is used as a fertilizer as an effective alternative for ammonium nitrate.
- It is used in the manufacturing of paper.
- It is used in the production of matches.
- It is used in the making of explosives.
Frequently Asked Questions – FAQs
What is potassium chlorate used for?
Potassium chlorate is used in chemical oxygen generators (also known as chlorate candles or oxygen candles), used as oxygen delivery systems, such as airplanes, space stations and submarines, and was responsible for at least one airplanes crashing.
What happens to potassium chlorate when heated?
Potassium chlorate decomposes to potassium chloride and to oxygen gas when heated strongly. In the presence of MnO2 as a catalyst the decomposition is faster.
What is the difference between potassium chloride and potassium chlorate?
Potassium chlorate is a useful oxidizer, and it is easy to use household chemicals to produce small quantities of it. The addition of potassium chloride moves ions, precipitating potassium chlorate out. For this reaction to work the boiling is needed; you can’t just let the bleach evaporate.
Is decomposition of potassium chlorate a redox reaction?
Potassium chlorate thermal decomposition is not excessive, it’s just a redox reaction. Disproportionation refers to the same product that functions both as an oxidizing agent and as a reduction agent, resulting in compounds that contain the same product in different oxidation states.
How does potassium chlorate decompose?
The thermal decomposition of potassium chlorate to obtain oxygen and potassium chloride. This reaction occurs at a temperature of between 150-300 ° C. For this reaction manganese(IV) oxide can be the catalyst.
Other important links:
| Sodium Chloride | Chlorine |


Comments