What is Potassium Nitrate (KNO3)?
KNO3 is a chemical compound with chemical name Potassium Nitrate.
Potassium nitrate also called saltpeter or niter, a white solid soluble in water formed by fractional crystallization of sodium nitrate and potassium chloride solutions. It occurs naturally as niter in rocks in India, South Africa and Brazil. When heated it decomposes to give the nitrite and oxygen. Unlike sodium nitrate it is non-deliquescent. Potassium nitrate is used in gunpowder, fertilizers and in the laboratory preparation of nitric acid.
Potassium nitrate is the most common desensitizing agent in over-the-counter dentifrices. At a concentration of 5%, potassium nitrate in conjunction with sodium or monofluorophosphate fluoride significantly reduces symptoms within 2 weeks of daily use. Potassium ions penetrate the length of the dentinal tubule and block repolarization of the nerve ending.
Frequent and regular application of a potassium nitrate dentifrice is necessary to avoid recurrence of symptoms, maintain a high abundance of extracellular potassium ions, and maintain the inter dental nerves in a hyperpolarized state. Potassium nitrate, often called saltpeter, occurs as an efflorescence in caverns and on soils in arid regions.
Synthesis of Potassium Nitrate (KNO3)
Potassium nitrate is a salt. It is prepared by neutralizing an acid. When potassium hydroxide neutralizes nitric acid potassium nitrate is formed.
KOH + HNO3 → KNO3 + H2O
Neutralizing nitric acid always makes “nitrate” salts. Other acids make other types of salts.
Potassium nitrate contains potassium (a soft, light, and silver metal), oxygen, and nitrogen (a colourless and odourless gas). It is an alkali metal nitrate because it is an ionic salt of potassium ions K+ ions and nitrate ions NO3−. It is solid white or sometimes white to dirty grey in colour. Potassium nitrate is soluble in hot water. This compound releases oxygen when heated or decomposed. It is a strong oxidizing agent It is widely used in the removal of the stump, fireworks, fertilizers, etc. It is a major constituent of black powder and food preservation techniques.
Properties of Potassium Nitrate – KNO3
| KNO3 | Potassium Nitrate |
| Molecular Weight/ Molar Mass | 101.1032 g/mol |
| Density | 2.109 g/cm3 |
| Boiling Point | 400 °C |
| Melting Point | 334 °C |
Potassium Nitrate structure (KNO3 Structure)
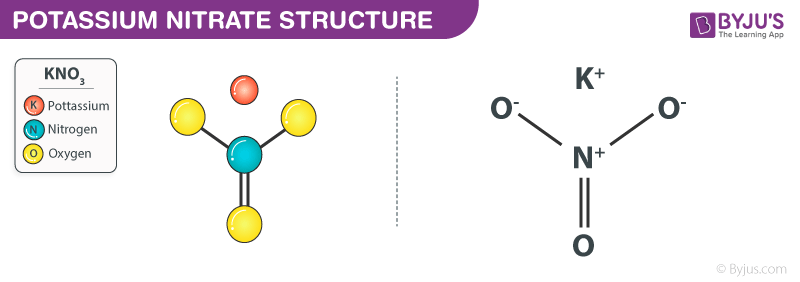
Potassium Nitrate Structure
Potassium Nitrate (KNO3 ) Uses
- It is used as a form of fertilizer as it contains all the macronutrients needed for the plants to grow.
- It is used as gunpowder in explosives such as bombs, grenades, etc.
- Used in the manufacturing and production of cigarettes.
- It is used extensively used in the preservation of hides
- It has medicinal applications such as a diuretic in medicine
- Used in toothpaste to make the teeth less sensitive to pain
- Used in the food industry to preserve meat against microbial agents
Potassium Nitrate (KNO3 ) Health Hazards
- Potential exposure – Potassium Nitrate is used in chemical analysis, as a food additive in fertilizers in medications as a vasodilator and as antidote for cyanide poisoning.
- Short term exposure – Potassium nitrate can affect when breathed in. Contact can cause eye and skin burns. Breathing the dust or mist can irritate the nose, throat and lungs and may cause coughing with phlegm. Higher exposures can cause pulmonary edema, a medical emergency that can be delayed for several hours. This can cause death.
- Long term exposure – Repeated skin contact causes dermatitis, drying and cracking. May cause lung irritation, bronchitis may develop. There is limited evidence that potassium nitrite may damage the developing fetus.
- Medical surveillance – If symptoms develop or overexposure is suspected, the following may be useful, blood test for methemoglobin. Lung function tests. Consider chest X-ray after acute overexposure.
- Potassium nitrate is an inorganic salt which has a molecular KNO3 formula. This is a common form of nitrate which has been used for numerous uses as a component, including agricultural preservatives, fertilizers, tree stump removal, rocket propellants, which fireworks.
- Potassium nitrate is a common active ingredient that exerts an anti-sensitive effect in toothpaste. It offers enhanced protection against the painful sensitivity of the teeth to ice, sun, acids, sweets or touch.
Frequently Asked Questions – FAQs
Is potassium nitrate harmful to humans?
A number of health hazards can present potassium nitrate. It can trigger breathing issues when inhaled, including coughing and shortness of breath. Contact with the skin or eye can lead to discomforts such as redness, itching, and pain.
What contains potassium nitrate?
Potassium nitrate is a nitric acid crystalline potassium salt. Many products in households, agriculture, and industry use potassium nitrate. For solar power plants, there are examples of toothpaste, fertilizers, fireworks, pesticides and molten salt.
Is potassium nitrate safe in toothpaste?
There is often confusion between nitrates and nitrites. The FDA recognizes nitrates used in potassium nitrate as secure and efficient for use in anti-sensitive dental products. Additionally, temporary pain relief is provided by delicate toothpaste.
What are the dangers of potassium nitrate?
Contact can trigger irritation of the eyes and skin. Potassium nitrate respiration may irritate the nose and throat causing sneezing and coughing. High concentrations may interfere with the blood’s capacity to carry oxygen that causes headache, tiredness, dizziness, and blue skin and lips.
What is potassium nitrite used for?
In the production of heat transfer salts, potassium nitrite is used. Potassium nitrite as a food additive E249 is a sodium nitrite-like preservative and is approved for use in the EU, USA, Australia and New Zealand.
Is potassium nitrate harmful to humans?
Potassium nitrate when breathed in will impact you. * Touch can cause discomfort to the eyes and skin. * Potassium nitrate for breathing can irritate the nose and throat causing sneezing and coughing.”
Is potassium nitrate a carcinogen?
Nither IARC nor the EPA have listed carcinogenicity nitrates. There are however several potential mechanisms that can metabolize nitrates to N-nitroso compounds, some of which are carcinogenic.
What plants benefit from potassium?
Potassium grows good lawns by encouraging deep-rooted lush, robust stems. By supporting solid stems and well-developed flowers it benefits roses and other flowering plants. The farmers depend on potassium to grow good crops. Plants which are rich in carbohydrates like potatoes need potassium to develop tuber.
Learn more about the Structure, physical and chemical properties of KNO3 from the experts at BYJU’S.
Other related links:
| Oxidizing Agent | Potassium Permanganate |


Comments