What are Soaps?
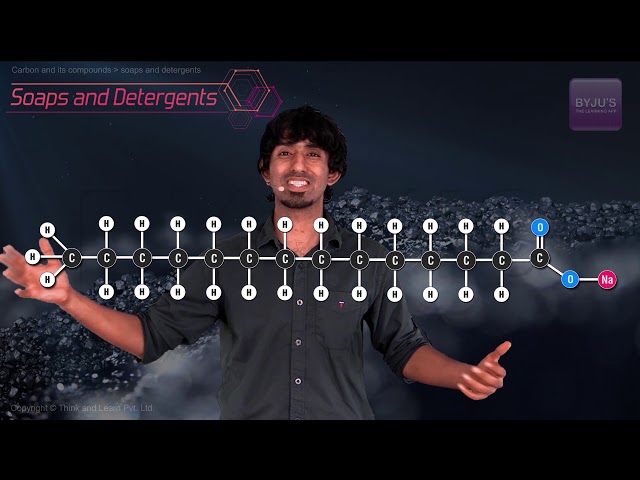
A soap is a water-soluble compound which is made via a process called saponification by the reaction between sodium hydroxide or potassium hydroxide with vegetable or animal oil (fats).
Characteristics of Soap
- Hardness – Harder soap which is a dense bar lasts longer.
- Cleansing – The first reason the majority of people use soap is to get clean. A soap molecule consists of a chain of carbon atoms where one end of the chain attracts oil and the other attracts water. Soap should be balanced and not too much or too less of cleansing ingredient should be added.
- Conditioner – Soap conditioners are referred to as emollients. Once you have washed your hands and what’s left behind on your skin after you rinse, depends on the type of soap a person uses. For instance, consider a person with dry skin, he/she should select a soap with moisturizing emollients that can prevent water evaporation.
- Lather – Most people like soap which produces lather. The balance of bubbles and cleansing, soothing cream makes lather so satisfying.
- Fragrance – It is an essential factor. Aromas evoke a unique combination of personal memory and enrich our daily life. Fragrances revitalize us, calm us, and most importantly mask our body odours.
What is Detergent?
Amphipathic molecules that contain charged hydrophilic or polar groups at the end of long lipophilic hydrocarbon groups are called detergents. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Detergents are also known as surfactants as they have the ability to decrease the surface tension of water.
Properties of Detergents
- The concentration at which micelles formation starts is called as critical micelle concentration (CMC).
- Aggregation number is the average number of monomers in a micelle.
- Relative micelle size is indicated by micelle molecular weight.
- The temperature at which the detergent solution is around or above its critical micelle concentration separates into two phases is called the cloud point.
Recommended Video
Cleansing Action of Soaps and Detergents
Most of the dirt is oily in nature and oil does not dissolve in water. The molecule of soap constitutes sodium or potassium salts of long-chain carboxylic acids. In the case of soaps, the carbon chain dissolves in oil and the ionic end dissolves in water. Thus, the soap molecules form structures called micelles. In micelles, one end is towards the oil droplet and the other end which is the ionic faces outside. Therefore, it forms an emulsion in water and helps in dissolving the dirt when we wash our clothes.
Soap is a kind of molecule in which both the ends have different properties.
- Hydrophilic end
- Hydrophobic end
The first one is the hydrophilic end which dissolves water and is attracted to it whereas the second one is the hydrophobic end that is dissolved in hydrocarbons and is water repulsive in nature. If on the surface of the water, soap is present then the hydrophobic tail which is not soluble in water will align along the water surface.
Micelles


In water, the soap molecule is uniquely oriented which helps to keep the hydrocarbon part outside the water. When the clusters of molecules are formed then hydrophobic tail comes at the interior of the cluster and the ionic end comes at the surface of the cluster and this formation is called a micelle. When the soap is in the form of micelles then it has the ability to clean the oily dirt which gets accumulated at the centre. These micelles remain as colloidal solutions. Therefore, the dirt from the cloth is easily washed away. The soap solution appears cloudy as it forms a colloidal solution which scatters light.
Read more about Soap and Detergents. We have briefly seen the properties of soaps and detergents, for any further query on this topic install BYJU’S learning app and enjoy an innovative approach to learning.
Read more:


Gr8??
Nice
thanks byjus
VERY USEFUL FOR EVERY STUDENT
Thanx byjus??
ethix
I simply wanted to write down a quick word to say thanks to you for those wonderful tips and hints you are showing on this site
It just awesome, i gained knowledge over this topic.
THANKS BYJUS