What is Sodium Nitrate?
Sodium nitrate is an inorganic nitrate salt of an alkali metal with the chemical formula NaNO3.
Commonly referred to as “Chile saltpeter”, this compound consists of a sodium cation (Na+) and a nitrate anion (NO3–). At room temperature, sodium nitrate exists as a white, crystalline solid which is highly soluble in water.
Table of Content
This compound is non-flammable. However, it is a strong oxidizing agent and can react with many flammable compounds violently. NaNO3 decomposes explosively when heated to temperatures above 538oC.

Rich deposits of sodium nitrate can be found in some South American countries such as Chile and Peru. The primary applications of this compound are in agriculture (fertilizers) and pyrotechnics.
Sodium Nitrate Structure
Sodium nitrate features an ionic bond between one Na+ ion and one NO3– ion. The structure of a NaNO3 molecule is illustrated below.
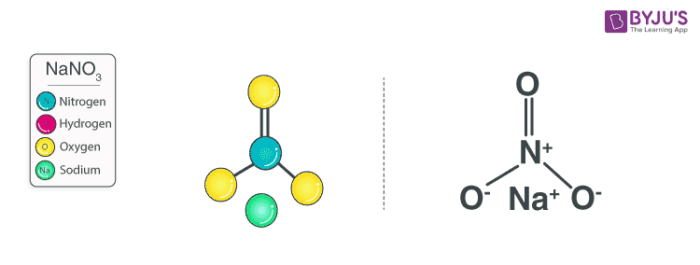
The nitrate anion has a trigonal planar structure in which 3 oxygen atoms are bonded to a central nitrogen atom. The negative charge on this ion is delocalized due to resonance. Therefore, the nitrogen atom a charge of +1 whereas each oxygen atom carries a charge of -⅔. The net formal charge on the NO3– is -1.
Sodium Nitrate Preparation
The industrial synthesis of sodium nitrate involves the neutralization of nitric acid with sodium carbonate, sodium bicarbonate, or sodium hydroxide. The chemical equations for these reactions are listed below.
- NaHCO3 + HNO3 → NaNO3 + H2O + CO2
- Na2CO3 + 2HNO3 → 2NaNO3 + CO2 + H2O
- NaOH + HNO3 → NaNO3 + H2O
It is important to note that the reaction between sodium hydroxide and nitric acid is a highly exothermic one (since NaOH is a strong base and HNO3 is a strong acid). Therefore, an alternate method of preparing sodium nitrate is by reacting sodium hydroxide with ammonium nitrate instead of nitric acid. The chemical reaction is:
NH4NO3 + NaOH → NH4OH + NaNO3
Alternately, ammonium nitrate can be reacted with sodium carbonate or sodium bicarbonate.
Na2CO3 + 2NH4NO3 → (NH4)2CO3 + 2NaNO3
NaHCO3 + NH4NO3 → NH4HCO3 + NaNO3
Properties of NaNO3
1. Chemical Data
| Sodium Nitrate | NaNO3 |
| Molar Mass | 84.99 grams per mole |
| Density | 2.257 grams per cubic centimetre |
| Melting Point | 308oC (581 K) |
| Boiling Point | 380oC (653 K, Decomposes) |
2. Physical Properties
- Sodium nitrate is a crystalline solid which is white.
- It has two crystal structures – rhombohedral and trigonal.
- This compound has a sweet odour.
- The solubility of NaNO3 in water corresponds to 91.2g/100mL at a temperature of 25o
- This compound is also highly soluble in ammonia.
3. Chemical Properties
- When dissolved in water, sodium nitrate dissociates into Na+ and NO3–
- It is a very strong oxidizing agent; it reacts violently with reducing agents.
- At high temperatures, this compound is known to explosively decompose.
Sodium Nitrate in Food
Sodium nitrate has been used in meat curing for centuries. Sodium nitrate has no antioxidant activity but becomes functional on reduction to nitrite. The important functions of sodium nitrite include stabilization of meat colour, texture improvement, development of the characteristics cured meat flavour, elimination of the problem of warmed over flavour and antimicrobial activity. Nitrites probably function as metal chelators may form nitroso compounds that have antioxidants properties and convert the heme proteins into stable nitric oxide forms.
Visible Influence of Sodium Nitrate on Plant Growth
Nitrogen is the first limiting element of plant growth in many of the soils. For this reason the application of any readily available nitrogenous fertilizer often materially increases crop growth. This effect following an application of sodium nitrate is often noted by the layman, with the result that in the past sodium nitrate has been looked upon by many as a stimulant. This is an erroneous conception and should be discreated.
How Sodium Nitrate Should be applied?
Because sodium nitrate is readily and entirely soluble in water, and because the nitrate ion is not absorbed by soil colloids, it should be applied for early utilization by the crop following application. This principle is applicable to all nitrate fertilizers. The loss of nitrates in drainage water is especially severe when nitrates are applied to sands and particularly sands with open subsoils, from which they may be leached easily.
Larger quantities of sodium nitrate may be applied to clays than the sands, but even in such cases the application of quantities greater than that which the crop is in position to utilize within a reasonable length of time should be avoided. When large amounts are to be used it will be found best to apply sodium nitrate in two or more applications. Such a practise will minimize the loss of nitrogen by leaching, and at the same time will avoid injuring plant roots by applying excessive quantities of soluble salt at one time. The first application may be broadcast or applied in the drill before plating, and the second and later applications may be applied as side dressings or top dressings. Top dressings of sodium nitrate often appear to be more effective during cool than during warm seasons.
Uses of Sodium Nitrate
Owing to its high solubility in water, low cost, and nitrogen content, sodium nitrate is used in several fertilizers. Some other uses of this compound are listed below.
- Hybrid forms of aqua regia can be prepared with the help of NaNO3. These hybrids also have the ability to dissolve gold.
- This compound is widely used as a food additive since it acts as a preservative.
- Sodium nitrate is used as an oxidizer in several types of fireworks.
- It is also a component of some instant cold packs.
- NaNO3 is one of the components used for the storage and transfer of heat in some solar power plants.
- In order to promote the growth of Nitrosomonas bacteria, this compound is added to the wastewater in several wastewater treatment plants.
Sodium nitrate is also used in several rocket propellants and is known to be a substitute for potassium nitrate in gunpowder.
Trivia
Sodium nitrate was referred to as ‘white gold’ in the 19th century. Wars have been waged over lands that were rich in sodium nitrate. The war of the Pacific, which was waged between 1879 and 1884, is commonly referred to as the saltpeter war. The South American country of Chile fought against Bolivia and Peru in order to obtain territory in the Atacama Desert (which was rich in sodium nitrate).
The development of the Haber process led to a decrease in the demand for saltpeter. This is because NaNO3 could now be produced synthetically with the help of ammonia. Eventually, the mining of NaNO3 from natural deposits became obsolete.
Frequently Asked Questions – FAQs
What is sodium nitrate used for?
Sodium nitrate is a kind of salt used for food preservation for a long time. It produces a separate flavour, regulates the oxidation of lipids and acts as an antimicrobial.
Can sodium nitrate explode?
Not combustible, but the material is a powerful oxidizer and may cause ignition due to its heat of response with reduction agents or fuels.
Is sodium nitrate carcinogenic?
Used as preservatives, sodium nitrate, and sodium nitrite add colour and flavour to processed meats. The International Cancer Research Agency (IARC) has evaluated and categorized ingested nitrates and nitrites as likely to cause cancer to humans.
When was sodium nitrate discovered?
Sodium Nitrate History. In 1820 or 1825, Chile Saltpeter’s first shipment to Europe arrived in England, but found no buyers and was dumped at sea to prevent customs tolls.
What is the difference between sodium nitrate and sodium nitrite?
Sodium nitrate is a salt often added to meats that are jerky, bacon and luncheon. On the other side, sodium nitrite is salt and antioxidant widely used for the healing of ham and bacon. Both chemicals function as food preservatives and, among other uses, add red or purple colour to processed meats.


Comments