What is Suspension?
A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it.
A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. In this type of mixture, all the components are completely mixed and all the particles can be seen under a microscope. A suspension is a heterogeneous mixture containing solid particles that are sufficiently large for sedimentation.
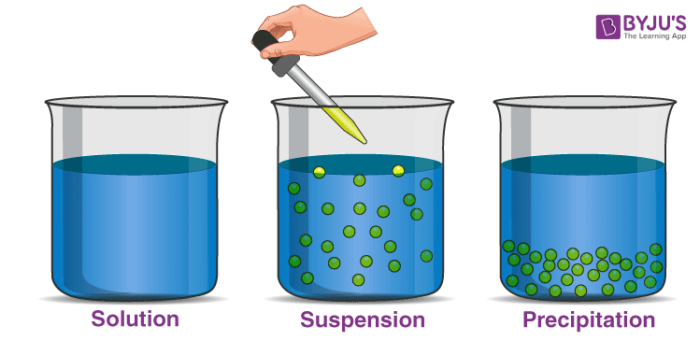
If we take a glass full of water and mix mud in it, it will form a heterogeneous mixture. We can easily identify the components of these mixtures. After some time we will observe that particles of mud settle down due to gravity. The particles in suspension are larger than the particles in a solution.
Recommended Videos

Properties of Suspension
- A suspension is a heterogeneous mixture.
- The size of solute particles in a suspension is quite large. It is larger than 100 mm in diameter.
- The particles of a suspension can be seen easily.
- The particles of a suspension do not pass through a filter paper. So a suspension can be separated by filtration.
- The suspension is unstable. The particles of a suspension settle down after some time.
- A suspension scatters a beam of light passing through it because of its large particle size.
Examples of Suspension
Some common examples of suspension are
- Muddy water
- Milk of magnesia
- Sand particles suspended in water
- Flour in water
- Slaked lime for whitewashing
- Paints in which dyes are suspended in turpentine oil.
What is a Solution?
A solution is a homogeneous mixture of substances. For example, when salt dissolves in water, a homogeneous mixture, or solution, forms. The component of a mixture that is present in the greatest quantity or that determines the state of matter of the solution is called the solvent and the other component is called solute.
What is Colloid?
A Colloid is an intermediate between solution and suspension. It has particles with sizes between 2 and 1000 nanometers. A colloid is easily visible to the naked eye. Colloids can be distinguished from solutions using the Tyndall effect. Tyndall effect is defined as the scattering of light (light beam) through a colloidal solution. The particles are termed as colloidal particles and the mixture formed is known as colloidal dispersion. Liquid, solid and gases all mix together to form a colloidal dispersion.
The different types of colloidal solution are:
- Aerosols: Solid or liquid mixed with gas; Example: fog (liquid in gas)
- Sols: Solid mixed with liquid; Example: Paint
- Emulsion: Liquid with liquid; Example: oil and water
- Gel: liquid in solid; Example: Fruit jelly
Difference between Colloid and Suspension
The difference between suspension and colloids are tabulated below.
| Suspension | Colloid |
| It is a form of the heterogeneous solution | It is a form of a homogeneous solution |
| Particle size greater than 1000 nm | Particle size range from 1 and 1000 nm |
| Particles settle down well | Particles do not separate |
| Can be separated by filtration | Cannot be separated by filtration |
| May scatter light | Shows Tyndall effect (scatters light) |
| Opaque | Translucent |
| Easily visible through the naked eye | Not visible through the naked eye |
To follow more about the difference between suspension and colloid, download BYJU’S – The Learning App.


excellent explanation