Catalyst is a common word that you might come across while studying chemistry especially while learning about chemical reactions. While some of the chemical reactions occur quickly, some take a long time and require extra materials or effort. This is where a catalyst comes in. What is the meaning of catalyst? We will find out in this lesson.
Table of Content
- Types of Catalysts
- Catalysis
- Heterogeneous Catalysis
- Homogeneous Catalysis
- Autocatalysis
- Frequently Asked Questions
What is Catalyst in Chemistry?
In Chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Most of the time a catalyst is used to speed up or increase the rate of the reaction. However, if we go to a deeper level, catalysts are used to break or rebuild the chemical bonds between the atoms which are present in the molecules of different elements or compounds. In essence, catalysts encourage molecules to react and make the whole reaction process easier and efficient.
Some of the important characteristic features of catalysts are,
- A catalyst does not initiate a chemical reaction.
- A catalyst does not be consumed in the reaction.
- Catalysts tend to react with reactants to form intermediates and at the same time facilitate the production of the final reaction product. After the whole process, a catalyst can regenerate.
A catalyst can be either solid, liquid or gaseous catalysts. Some of the solid catalysts include metals or their oxides, including sulphides, and halides. Semi-metallic elements such as boron, aluminum, and silicon are also used as catalysts. Likewise, liquid and gaseous elements which are in pure form are used as catalysts. Sometimes, these elements are also used along with suitable solvents or carriers.
The reaction which involves a catalyst in their system is known as a catalytic reaction. In other words, catalytic action is a chemical reaction between the catalyst and a reactant. This results in the formation of chemical intermediates that can further react quite readily with each other or with another reactant to form a product. However, when the reaction between the chemical intermediates and the reactants occurs or takes place the catalyst is regenerated.
The reaction modes between the catalysts and the reactants usually tend to vary widely and in the case of solid catalysts, it is more complex. Reactions can be acid-base reactions, oxidation-reduction reactions, coordination complexes formation as well as production of free radicals. For solid catalysts, the reaction mechanism is greatly influenced by surface properties and electronic or crystal structures. Some types of solid catalysts such as polyfunctional catalysts can have several reaction modes with the reactants.
Also Read: Chemical Kinetics
Brief History
If we look at the general meaning of catalyst it is anything that increases the rate of a process. Catalyst is a term derived from Greek καταλύειν, meaning “to annul,” or “to unite,” or “to pick up.” Meanwhile, the concept of catalysis was first researched by chemist Elizabeth Fulhame and it was described in her book in the year 1794. This book content was based on her work in oxidation-reduction experiments.
The first chemical reaction in organic chemistry that utilized a catalyst was studied in 1811 by Gottlieb Kirchhoff who was a Russian chemist of German origin. The term catalysis was later used by a Swedish chemist named Jöns Jakob Berzelius in 1835 to describe reactions that were sped up by certain substances. The substances further remained unchanged after the reaction.
Types of Catalysts With Examples
There are several types of catalysts that can be used depending on the need or requirement of the chemical reaction. They are as follows;
Positive Catalysts
Catalysts that increase the rate of a chemical reaction are positive catalysts. It increases the rate of reaction by lowering the activation energy barriers such that a large number of reaction molecules are converted into products, thereby the percentage of yield of products increases.
Positive catalyst example: In the preparation of NH3 by Haber’s process Iron oxide acts as a positive catalyst and increases the yield of ammonia in spite of less reaction of Nitrogen.
Negative Catalysts
Catalysts that decrease the rate of reaction and negative catalyst. It decreases the rate of reaction by increasing the activation energy barrier which decreases the number of reactant molecules to transform into products and hence the rate of reaction decreases.
Negative catalyst example: Decomposition of Hydrogen peroxide into water and oxygen is retarded by using Acetanilide, this acts as a negative catalyst to decrease the rate of decomposition of hydrogen peroxide.
Promoter or Accelerators
A substance that increases the catalyst activity is known as a Promoter or accelerator.
Example: In Haber’s process molybdenum or a mixture of potassium and Aluminium oxides act as Promoters.
Catalyst Poisons or Inhibitors
Substances that decrease the catalyst activity are known as catalyst poisons or inhibitors.
Example: In the hydrogenation of alkyne to an alkene, catalyst palladium is poisoned with barium sulphate in quinolone solution and the reaction is stopped at alkene level. The catalyst is known as Lindler’s catalyst.
Units
The derived SI unit for measuring the catalytic activity of a catalyst is “katal”. It is further quantified in moles per second. If we were to describe the productivity of a catalyst it can be defined by the turnover number (or TON). Catalytic activity can be described by the turn over frequency (TOF) which is TON per time unit. Likewise, the enzyme unit is its biochemical equivalent.
Also Read: Enzyme Catalyst
Catalysis
When a catalyst is used to increase the rate of a chemical reaction this phenomenon is known as catalysis.
What are the Types of Catalysis?
On the basis of nature and the physical state of substance employed in the chemical reaction, catalysis is of three types;
- Homogeneous catalysis
- Heterogeneous catalysis
- Autocatalysis
Heterogeneous Catalysis
In this type of catalysis, the reacting substances in a reaction and catalyst employed in that reaction are not in the same state of matter.
Examples 1: Preparation of Ammonia by Haber’s process.
Pure and dry Nitrogen and Hydrogen gases in 1 : 3 ratio are passed through a compressor where high pressure of 200 – 30 atmosphere is maintained. In this process, Iron oxide is used as a catalyst. It is solid oxide employed in a process where the reactants are in a gaseous state. The Nitrogen (g) reacts with hydrogen (g) to form Ammonia (g), in the pressure of Iron oxide solid thus it is heterogeneous catalysis
Example 2: Manufacture of sulphuric acid by Contact process.
In this process, the oxidation of sulphur dioxide is a major step. In this oxidation, sulphur dioxide is a gas and oxygen is a gas while vanadium pentoxide is a solid catalyst. In this process, reactants and catalysts are in different states of matter.
Mechanism of Heterogeneous Catalyst
Heterogeneous catalysis involves both adsorption as well as intermediate compound formation. The reactant molecule gets adsorbed on the activation centre of the surface of the catalyst. These combine to form an activated complex which is an intermediate compound. This compound decomposes to give products.
As soon as the products are formed these get desorbed from the surface without any lapse in time. The heterogeneous catalysis involves initially adsorption of reactants on the surface of catalyst, Intermediate compound formation, dissociating into a product.
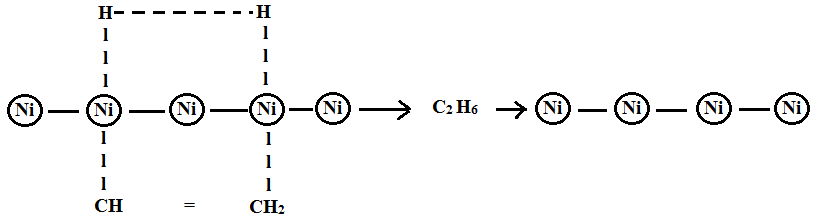
Example: Hydrogenation of ethene into ethane on the surface of the nickel.
- Ether and hydrogen molecules are adsorbed on the surface of the catalyst.
- Hydrogen occupies most of the activation centre and is known as occlusion.
- Ethane molecules attack at their double bond region to form an activated complex.
- Ether reacts with active hydrogen to form ethane.
- This ethane gets desorbed on the surface of the catalyst.
Electrocatalysts
In electrochemistry especially when we are dealing with fuel cell engineering, several types of metal-containing catalysts are used. These catalysts have one main role which is to enhance the rates of the half-reactions that occur in a fuel cell. A popular electrocatalyst used in a fuel cell is most of the time based upon nanoparticles of platinum. Now, these are supported on slightly larger carbon particles. As this catalyst comes in contact with one of the electrodes in a fuel cell, platinum increases the rate of oxygen reduction either to water or hydroxide (also hydrogen peroxide).
Homogeneous Catalysis
The catalysis in which the catalyst employed in the reaction and the reactants are in the same state of matter, that process is referred to as homogeneous catalysis. In the same step as the reactants, homogeneous catalysts work. Usually, homogeneous catalysts with substrates are dissolved in a solvent. The effect of H+ on the esterification of carboxylic acids, such as the formation of methyl acetate from acetic acid and methanol, is one example of homogeneous catalysis. Hydroformylation, hydrosilylation, hydrocyanation involve high-volume processes requiring a homogeneous catalyst. Homogeneous catalysis is frequently synonymous with organometallic catalysts for inorganic chemists. However, many homogeneous catalysts are not organometallic, as demonstrated by the use of cobalt salts that catalyze the oxidation of p-xylene to terephthalic acid.
Whereas in the study of catalysis, transition metals often attract much of the focus, small organic molecules without metals may also exhibit catalytic properties, as is evident from the lack of transition metals in many enzymes. Organic catalysts normally need a higher load (catalyst quantity per unit quantity of reactant, expressed in mol percentage of substance) than transition metal(-ion)-based catalysts, but these catalysts are generally commercially available in bulk, helping to reduce costs. Such organocatalysts were considered a “new breed” in the early 2000s and are competitive with conventional catalysts containing metal(-ion).
Example 1: Hydrolysis of ethyl acetate in the presence of dilute acid.
Ethyl acetate is a liquid that contains an ester functional group. It reacts with water in the presence of dilute sulphuric acid which is a liquid to give ethyl alcohol and acetic acid.
In the above reaction reactants and catalysts are in the same state of matter. Hence, it is homogeneous catalysis
Example 2: Oxidation of sulphur dioxide in the lead chamber process.
Lead chamber process is used in the manufacture of sulphuric acid. In this process, Nitric oxide gas is used as catalysis.
In the above reaction, SO2 and O2 along with catalyst NO is also a gas hence it is homogeneous catalysis.
Also Read: Wilkinson’s Catalyst
Mechanism of Homogeneous Catalysis
The homogeneous catalysis takes place by intermediate compound formatter theory.
Let us consider the oxidation of SO2 into SO3 by the lead chamber process. In this nitric oxide gas is the catalyst.
This NO reacts with SO2 to form SO2 and “NO2” as an intermediate compound.
First step: Nitric oxide combines with oxygen to form nitrogen dioxide (NO2). This NO2 acts as an intermediate compound, which reacts with SO2 to form sulphur trioxide and NO
2NO(g) + O2(g) → 2NO2(g) Intermediate compound
2SO2 + 2NO2 → 2SO3(g) + 2NO(g)
Photocatalysts
Photocatalysis is the phenomenon wherein the catalyst is able to receive light (such as visible light) and be promoted to an excited state.
Autocatalysis
In the autocatalytic reaction, there is no specific catalyst that is added. Instead, one of the products acts as a catalyst and increases the rate of formation of products.
Example 1: Decomposition of Arsene (AsH3) is formed by the Arsenic formed in the reactor is “autocatalyst”.
2As H3 → 2As + 3H2
In this process As acts as a catalyst.
Example 2: Oxidation of Oxalic acid by KMnO4
When Permanganate is added to acidic solution oxidation of oxalate ions (or oxalic acid) occur. The reaction results in the formation of auto-catalyzes the reaction. The rate of reaction between Potassium permanganate and acidified oxalate solution is initially slow. ions that are formed during the reaction helps in increasing the rate of reaction.
Frequently Asked Questions On Catalyst
How can a positive catalyst alter the reaction?
A positive catalyst is to make reaction rate very first by changing the path of reaction by decreasing the activation energy basis. Such that a large number of reactant molecules are converted into products.
What is the role of catalyst poison in Rosenmund reaction?
In the Rosenmund reaction aldehyde is prepared by reducing Acid halides with hydrogen gas in the presence of palladium. If a catalyst is not poisoned the reaction is not stopped at aldehyde level which is feather reduces of alcohol. In order to stop at the aldehyde level. Palladium is poisoned with Barium sulphate.
What are the key factors in heterogeneous catalysis?
In heterogeneous catalysis, the reacting and catalyst are in different states of matter. The most important steps in this process are;
– Adsorption of reactant molecules activation centre.
– Formation of activation complex at the centre.
– This complex decomposes to give products.
– Desorption of products from the surface of the catalyst.
What is the role of promoters in Haber’s process?
Promotors or accelerators increase the catalyst activity in a process. In Haber’s process of manufacturing Ammonia, Nitrogen reacts with hydrogen to form NH3. Nitrogen is very less reactive and the yield of Ammonia is very less, to increase the percentage yield of Ammonia formed NO is used as a promoter.
What is the significance of autocatalysis?
Auto catalysis is self-catalysis in this process one of the products formed acts as a catalyst and increases the reaction rate.


