Emulsions are the mixtures of two or more type of liquids where, one is such as droplets, of tiny or even ultramicroscopic size, which are distributed throughout each other. These are usually formed from the component of liquids either in natural form or, more often, using mechanisms such as the agitation, which is provided that these fluids mixed have no kind of mutual solubility.
Emulsions are said to be stabilized by some agents forming films at the surface of droplets or those which impart to them a kind of mechanical stability. The unstable form of emulsions eventually separates into two forms of liquid layers. The stable emulsions are destroyed by destroying or by deactivating the emulsifying agent—for example by the addition of appropriate third party substances or even by the process of freezing or by heating.
Some common emulsions are milk (where the dispersion of fat molecules or droplets in the aqueous solution) and also butter (which is the dispersion of droplets of particles of an aqueous solution in the fat).
Table of Content
- What is Emulsion?
- Emulsion Examples
- Properties of Emulsion
- Types of Emulsions
- Emulsifier
- How do Emulsifiers Work?
- Identifying Types of Emulsions
- Uses of Emulsions
What is Emulsion?
An emulsion can be defined as a colloid consisting of two or more non-homogenous type of liquids wherein one of the liquid contains the dispersion of the different form of liquids.
Emulsion Examples
Emulsions basically consist of a dispersion of two liquids that are immiscible with each other. One of the liquids act as the dispersion medium and the other will act as the dispersed phase. In simple words, emulsions are colloids in which both the dispersed phase and dispersion medium are liquids. Oil and the mixtures of water are the emulsions when are shaken together. The oil forms drop and then disperses throughout the water.
The term emulsion is also applied to a group of mixed systems called as solutions, or gels or suspensions. Take, For example, the photographic emulsion is a gelatin gel consisting of tiny crystals dispersed in it. Some other examples of emulsions include butter which is an emulsion of water in fat and egg yolk containing lecithin.
| DISPERSED PHASE | DISPERSION MEDIUM | TYPE OF COLLOID | EXAMPLE |
| Solid | Solid | Solid | Some Coloured Glasses And Gemstones |
| Solid | Liquid | Solid | Paints, Cell Fluids |
| Solid | Gas | Aerosol | Smoke, Dust |
| Liquid | Solid | Gel | Cheese, Butter, Jellies |
| Liquid | Liquid | Emulsion | Milk, Hair Cream |
| Liquid | Gas | Aerosol | Fog, Mist, Cloud, Insecticide Sprays |
| Gas | Solid | Solid | Pumice Stone, Foam Rubber |
| Gas | Liquid | Foam | Froth, Whipped Cream, Soap Lather |
Properties Of Emulsions
- Emulsions contain both a continuous and the dispersed with the boundary coming between the phases that are called “interface”.
- Emulsions have a cloudy appearance due to many phase interfaces scattering light passing through the emulsions.
- Emulsions appear in white colour when the light is dispersed in equal proportions.
- If the emulsion is dilute, then higher-frequency and the low-wavelength type of light will be scattered in more fractions, and this kind of emulsion will appear in blue in colour. This is also referred to as the Tyndall effect.
Also Read: Tyndall Effect and Dispersion of Light
Types of Emulsion
Emulsions can be classified on the basis of the properties of the dispersed phase and the dispersion medium.
1) Oil in water (O/W):
In this type of emulsion, the oil will be the dispersed phase and water will be the dispersion medium. The best example for o/w emulsion is milk. In milk, the fat globules (which act as the dispersed phase) are suspended in water (which acts as the dispersion medium).
2) Water in oil (w/o):
In this type, water will be the dispersed phase and oil will be the dispersion medium. Margarine (a spread used for flavouring, baking and working) is an example of water in oil emulsion.
Emulsifier / Emulsifying agent
These are substances which are added to emulsions for stabilization purpose. The various characteristics of emulsifiers are given below:
- They are substances which have a hydrophilic end (polar) as well as a hydrophobic end (non-polar).
- They are soluble in both water and oil.
- Emulsifiers form a layer between the dispersed phase and the dispersion medium, thereby preventing the dispersed phase particles to come together to form larger particles and separate out.
- Emulsifiers can be cationic, anionic or even non-polar.
- It is not just the percentage of water and oil which decides whether it is oil-in-water or a water-in-oil emulsion. On the other hand, it depends on which among water and oil can solvate the emulsifier to a larger extent.
- If the emulsifier is more soluble in water than the water becomes the dispersion medium and oil becomes the dispersed phase and hence we get oil in water emulsion.
- On the other hand, if the emulsifier is more soluble in oil, then oil becomes the dispersion medium and water becomes the dispersed phase.
- The commonly used emulsifiers for o/w emulsions are proteins, gums, soaps etc.
- The commonly used emulsifiers for w/o emulsions are heavy metal salts of fatty acids, long-chain alcohols etc.
Some common examples of emulsifiers are egg yolk, mustard, sodium phosphates, DATEM, Mono- and diglycerides, cellulose, soy lecithin, etc.
How do Emulsifiers Work?
To understand this, firstly we need to understand the process of coalescing. Coalescing is the process in which the similar particles in the emulsions come together to form larger and bulkier particles leading to the separation of the dispersed phase and dispersion medium.
Emulsifiers help in preventing coalescing by forming a physical barrier between the dispersed phase and dispersion medium. As we have seen before emulsifiers, like soap, has both a hydrophilic end and a hydrophobic end. Hence, they can attach to both polar and non-polar substances. Let us take the example of sodium stearate. C17H35COO–Na can be represented as;
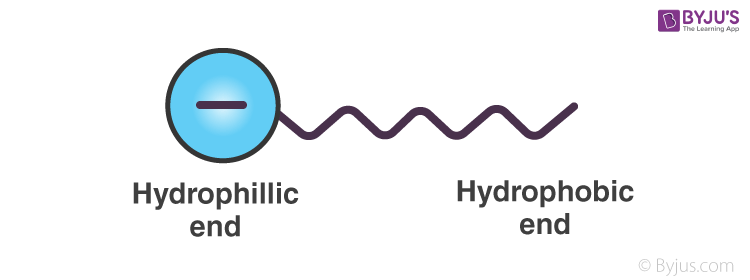
When this is added to an o/w emulsion, molecules of C17H35COO– surround the oil droplet with their non-polar tails/hydrophobic end (the hydrocarbon chain) extending into the oil & their polar heads/hydrophilic end (the carboxylate ion) facing the water as given in the figure.
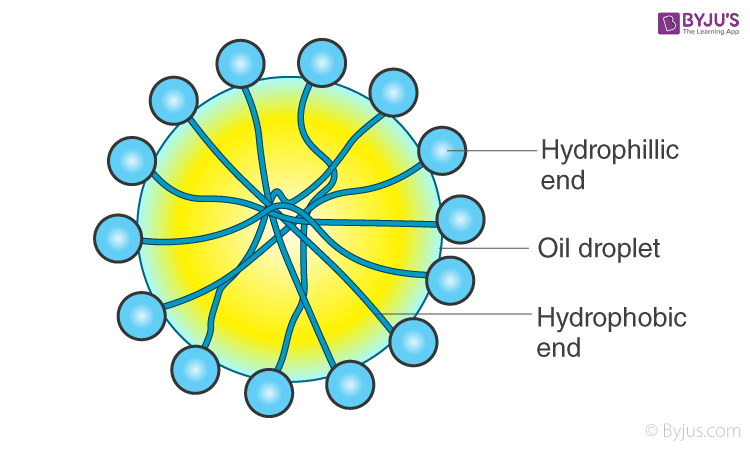
This arrangement brings a stronger adhesive force between the oil (dispersed phase) and water (dispersion medium). This new formed adhesive force will be more than the cohesive force between oil – oil and water – water. Hence, oil particles will not have the tendency to come together to form larger particles. This helps in preventing coalescing, thereby stabilizing the emulsion.
Note:
For w/o emulsion, the orientation of the emulsifier would be the opposite as that of o/w. i.e. non-polar (hydrophobic end) tail extends outside and polar (hydrophilic end) head faces inwards.
Theories of Emulsification
Since there are different processes and mechanisms (both chemical and physical) involved in the process of emulsification, there are several theories that accompany it.
Surface Tension Theory: This theory states or describes emulsification as a process that occurs by the reduction of interfacial tension between two phases.
Repulsion Theory: With this theory, we learn that the emulsifying agent produces a film over one phase which further leads to the formation of globules. These compounds tend to repel each other and the repulsive force that exists between them help them to remain suspended in the dispersion medium.
Also Read: Thin Film Interference
Methods to Identify the Type of Emulsions
1) Dilution test
On adding water to an o/w emulsion, it will still remain stable as water is the dispersion medium, but on adding oil it will get destabilized as oil & water are immiscible. Similarly, w/o emulsion can be diluted with oil & would still be stable, but would get destabilized on the addition of water.
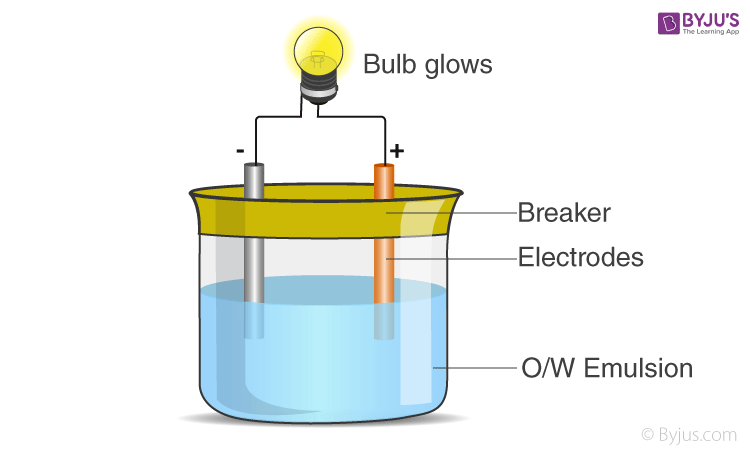
2) Conductivity test
In this test the emulsion is kept between 2 electrodes and a bulb is connected in the circuit as shown in the diagram. An o/w emulsion will conduct electricity as water conducts electricity, but a w/o will not conduct electricity.
3) Dye test
In this, a water-soluble dye is added to the emulsion. If it is an o/w emulsion, the dispersion medium appears red and the dispersed phase colourless and vice-versa.
Separation of Emulsions
The different methods by which emulsions can be separated into its constituent liquids include;
1) Heating
2) Centrifuging
Applications and Uses of Emulsion
Emulsions are very much famous in various fields of science. It is utilized in the tanning and dyeing industries, used in the manufacturing process of plastics and synthetic rubber.
- Usually used in cosmetics, pharmaceuticals, personal hygiene.
- Microemulsions are used to deliver vaccines to kill various microbes.
- It is used in chemical synthesis mainly in the manufacture of polymer dispersions.
- It is used in firefighting.
- Nanoemulsions such as soybean oil are used to kill microbes.
- Mayonnaise is an oil in water emulsion with egg yolk or sodium stearoyl lactylate.


