Quantum numbers are numbers allocated to all the electrons in an atom and they describe certain characteristics of the electron. The characteristics of the orbital are used to define the state of an electron completely and are expressed in terms of three numbers as Principal quantum number, Azimuthal quantum number and Magnetic quantum number and Spin Quantum number. In this piece of article, we will be discussing Azimuthal quantum number.
Table of Content
- Azimuthal Quantum Number Definition
- History of Azimuthal Quantum Number
- Subsidiary Quantum Number
- Angular Momentum Quantum Number
- Frequently Asked Questions-FAQs
What is Azimuthal Quantum Number?
The quantum number associated with the angular momentum of an atomic electron.
It is also termed as the orbital angular momentum quantum number, orbital quantum number or second quantum number, and is symbolized as ℓ. This number describes the shape of the orbital and also determines the orbital angular momentum. An example of the angular quantum momentum number would be a p orbital that is associated with an azimuthal quantum number equal to 1.
History
Arnold Sommerfeld posited the term azimuthal quantum number from the Bohr model of the atom. The Rutherford-Bohr model or Bohr model, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus – similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity.
The Bohr model has its existence from spectroscopic analysis of the atom in combination with the Rutherford atomic model. Angular Momentum was found to be ‘0’ at the lowest level of quantum. Orbits with zero angular momentum were termed as ‘pendulum’ orbits.
Subsidiary Quantum Number
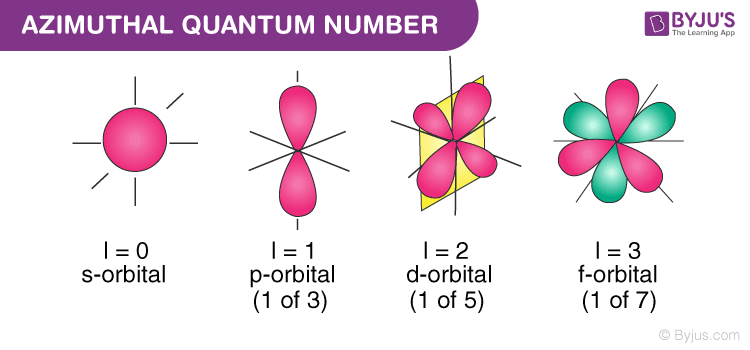
Azimuthal quantum number describes the shape of orbital. It is denoted by . Values of are from zero to n-1.
For s-orbital, ℓ = 0
For p-orbita, ℓ = 1
For d-orbital, ℓ = 2
For f-orbital, ℓ = 3
With the help of the value of azimuthal quantum number we can determine the total number of energy sub-levels in a given energy level.
Angular Momentum Quantum Number
- Intrinsic (or spin) angular momentum quantum number, or simply spin quantum number
- Orbital angular momentum quantum number (the subject of this article)
- Magnetic quantum number, related to the orbital momentum quantum number
- Total angular momentum quantum number.
Related Links:

If you wish to learn more physics concepts with the help of interactive video lessons, download BYJU’S – The Learning App.
Frequently Asked Questions – FAQs
What is the range of Azimuthal Quantum number?
The range of Azimuthal Quantum number is between 0 to n-1.
What is the value of the spin quantum number?
The value of spin quantum number is 2.
For a quantum number, if n=4, what is the value that is not equal to l?
There are three quantum numbers: n, l, and m. The principal quantum number is n. According to the questions, the principal quantum number, n = 4. Therefore, l, which is the angular quantum number, can have the values 0,1,2,3 and not beyond 3.
Name the quantum number that describes the size of the orbital.
Principal quantum number describes the size of the orbital. The principal quantum number cannot be zero, and the size of the orbit increases with increase in numbers.
What are the 4 types of quantum numbers?
The 4 types of quantum numbers are principal quantum number, angular momentum, magnetic moment, and spin quantum number.




It was useful