What is Electric Dipole?
An electric dipole is defined as a couple of opposite charges q and –q separated by a distance d. By default, the direction of electric dipoles in space is always from negative charge -q to positive charge q. The midpoint q and –q is called the centre of the dipole. The simplest example of an electric dipole is a pair of electric charges of two opposite signs and equal magnitude separated by distance.
Visualizing Electric Dipole
Suppose there are two charges of equal magnitude ‘q’, separated by a distance, d. Let the first charge be negative and the second charge is positive. This combination can be called an electric dipole. Therefore, we can say that an electric dipole is created by the combination of equal and opposite charges by a separation of a certain distance.
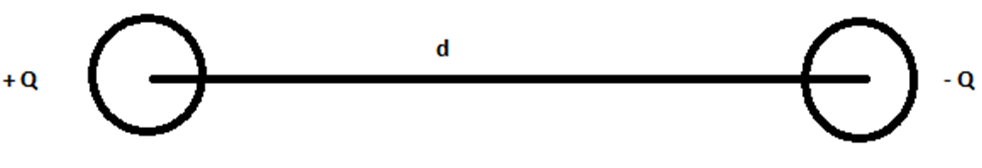
An electric dipole is denoted by the symbol
The magnitude of an electric dipole is given as:
\(\begin{array}{l} \overrightarrow{p} \end{array} \)=\(\begin{array}{l} Q \overrightarrow {d} \end{array} \)
Direction of Electric Dipole Moment
You may also want to check out these topics given below!
- Electric Potential Of A Dipole And System Of Charges
- Torque on an Electric Dipole in a Uniform Electric Field
- Magnetic Dipole Moment
Electric potential due to a Dipole (V)
Suppose there are two charges –q, placed at A, and +q placed a B, separated by a distance d, forming a dipole. Suppose the midpoint of AB is O.
The Electric potential due to a dipole at any point P, such that OP = r will be:
\(\begin{array}{l}\frac{1}{4\pi \epsilon } \frac{p cos \theta }{r^{2}}\end{array} \) |
Case 1: If θ = 90°
Case 2: If θ = 0°
\(\begin{array}{l}\frac{1}{4\pi \epsilon } \frac{p }{r^{2}}\end{array} \) |
Physical Significance of Dipole
Electric dipole is not only prominent in electrostatics but also in chemistry. In most molecules the centre of positive and negative charges coincide at the same point because of which the distance between two charges is zero. Carbon dioxide and methane fall under the category of zero dipole moment. This type of molecules are known as non-polar molecules. The molecules that have permanent dipole moment as the centre of positive and negative charge don’t coincide are called polar molecules.
If you would like to read more about polar and non-polar molecules, click on the link below:
Frequently Asked Questions – FAQs
What is the net force acting on a dipole placed in a uniform electric field?
The forces on the two charges constituting the dipole are equal and opposite. Hence, the net force is zero.
What is the SI unit of the dipole moment?
The SI unit of dipole moment is Coulomb.meter
When is the torque on a dipole maximum?
When the dipole is held perpendicular to the field, the torque is maximum.
When is the torque on a dipole minimum?
When the dipole is parallel to the field, the torque on a dipole is minimum.
Give an example of the electric dipole.
A pair of electric charge of two opposite signs and equal magnitude separated by a distance.
Stay tuned with BYJU’S to learn more Physics concepts with the help of interactive video lessons.




Super ? explanation