What are the Properties of Gases?
Gasses do not possess any definite volume or shape. They totally fill all the space accessible to them. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. This autonomy of movement of gaseous molecules is because of the very weak binding forces amidst molecules. In other words, their intermolecular forces are very weak. Because of this, the molecules of a gas are in a continuous motion and are related to high velocity and therefore high kinetic energy. The properties are given below:

1. Compressibility
Particles of gas have huge intermolecular spaces in the midst of them. By the exertion of pressure, much of this space can be diminished and the particles are brought closer. Thus, the volume of gas can be hugely reduced. This is termed as compressing the gas (Figure).
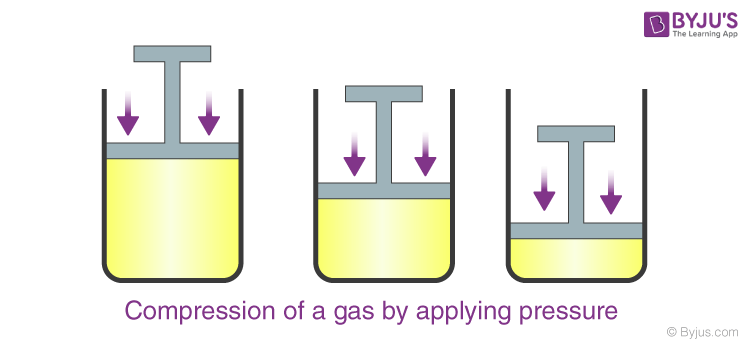
When we increase the pressure from 1 atmosphere to 2 atmospheres, the volume of gas gets compacted to half whereas if the volume of water was made to decrease similarly, it would reduce only by 0.00001 parts.
Decreasing the temperature can also decrease the volume of a gas. When the temperature is reduced, there is a lesser amount of energy in the particles; their mobility is diminished and they move less away from each other. As a consequence, the intermolecular pull becomes more prominent and the particles come closer. This decreases the volume of the gas.
2. Expansibility
When pressure is exerted on gas, it contracts. On the other hand, when pressure is freed, the gas expands.
When the temperature is augmented, the constituent particles gain more energy, travel faster and move away from each other. Consequently, the intermolecular pull becomes less prominent. The gas’s volume increases.
3. Diffusibility
The molecules of the gas are in perpetual motion, at a very high velocity. There is a huge amount of intermolecular space amid the molecules. When two gases are mixed, particles of one gas can effortlessly pass through the intermolecular space of the other gas. As an outcome both the gases get completely and consistently mixed. Thus, a mixture of gases at all times remains homogeneous.
4. Low Density
Since gases have large intermolecular spaces, they have very large volumes when compared to their mass. Therefore, they have less densities. If 1 ml of water at 39.2oF is transformed into steam at 212oF and 1-atmosphere pressure, it will occupy a volume of 1700 ml.
5. Exertion of Pressure
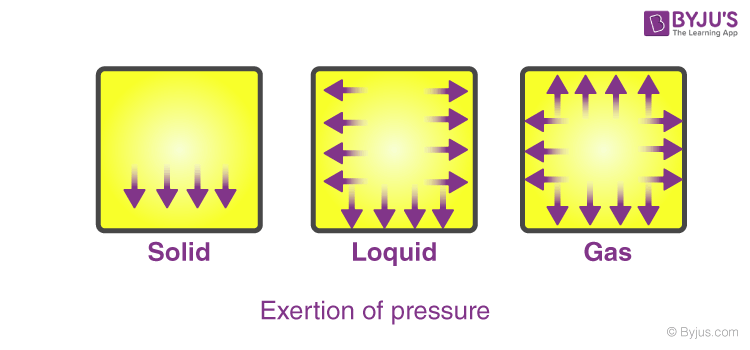
Solids exert pressure only in the downward direction. Liquids apply pressure downward as well as to the sides. But gases apply pressure in all directions (a good sample is a balloon). This pressure is because of the bombardment of the particles against the walls of the vessel (Figure).
If you wish to learn more physics concepts with the help of interactive video lessons, download BYJU’S – The Learning App.




The video is very helpful