Rydberg formulaLyman seriesBalmer seriesPaschen seriesBrackett seriesPfund seriesBrackett seriesHumphreys series
Spectral series are the set of wavelength arranged in a sequential fashion. Which characterises light or any electromagnetic radiation emitted by energised atoms.

Figure(1): Spectrum of Hydrogen gas along with spectral series and respective wavelength.
Hydrogen atom is the simplest atomic system found in nature, thus it produces the simplest of these series. When the beam of light or any radiation is made to enter the device through a slit, each individual component of the light or radiation form images of the source. These images can be visualised when resolved under the spectroscope. The images got will be in the form of parallel lines arranged next to each other with regular spacing. The lines will be apart in higher wavelength side and they come closer gradually when moved from higher to lower wavelength side. The shortest wavelength will possess least spaced spectral lines and it is named as series limit.
Rydberg formula
Atomic hydrogen displays emission spectrum. This spectrum enfolds several spectral series. Once the electrons in the gas are excited, they make transitions between the energy levels. These spectral lines are the consequence of such electron transitions between energy levels modelled by Neils Bohr. The wavelengths of the spectral series is calculated by Rydberg formula.
Rydberg formula relates to the energy difference between the various levels of Bohr’s model and the wavelengths of absorbed or emitted photons. It is mathematically expressed as-
Where,
- ? is the wavelength
- R is the Rydberg constant has the value 1.09737✕107 m-1
- Z is the atomic number
- nl is the lower energy level
- nh is the higher energy level
Note that this equation is valid for Hydrogen and Hydrogen like elements. The equation returns meaningful value only when nh > nl. In the development of quantum mechanics, the classification of series by Rydberg formula plays a major role. They are also prime concepts in astronomical spectroscopy. Especially in the detection of Hydrogen and in calculating Red shift.
How Spectral Series are Formed
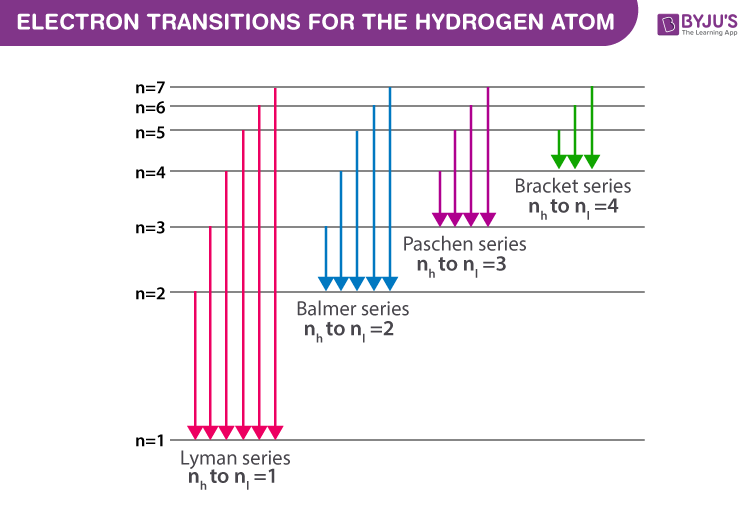
Figure(2): Electron transitions inside a Hydrogen atom
Every atom enfolds set of energy levels/states which is modelled and well explained through Bohr’s atomic model. He names energy states using quantum numbers(n=1,2,3,4,5,6…..). When electrons jump from higher energy states(nh) to lower energy state(nl), a photon of energy nh – nl is emitted. As the energy corresponding to each state are fixed, the difference between the energy states are also fixed thus the transition between similar energy states will produce the photon of the same energy.
The spectral series is broken into corresponding series based on the electron transition to lower energy state. The Greek alphabets are used within the series to segregate the spectral lines of corresponding energy. The spectral series of Hydrogen are:
Lyman series (nl=1)
The series was discovered during the years 1906-1914, by Theodore Lyman. Thus it is named after him. According to Bohr’s model, Lyman series is displayed when electron transition takes place from higher energy states(nh=2,3,4,5,6,…) to nl=1 energy state. All the wavelength of Lyman series falls in Ultraviolet band.
Refer to the table below for various wavelengths associated with spectral lines:
| Energy level (n) | Wavelength (? in nm) in vacuum |
| ∞ | 91.175 |
| 6 | 93.78 |
| 5 | 94.974 |
| 4 | 97.256 |
| 3 | 102.57 |
| 2 | 121.57 |
Balmer series (nl=2)
The series was discovered during the years 1885, by Johann Balmer. Thus the series is named after him.
Balmer series is displayed when electron transition takes place from higher energy states(nh=3,4,5,6,7,…) to nl=2 energy state. All the wavelength of Balmer series falls in visible part of electromagnetic spectrum(400nm to 740nm). In astronomy, the presence of Hydrogen is detected using H-Alpha line of the Balmer series, it is also a part of the solar spectrum.
Refer to the table below for various wavelengths associated with spectral lines.
| Energy level (n) | Wavelength (? in nm) in air |
| ∞ | 364.6 |
| 7 | 397.0 |
| 6 | 410.2 |
| 5 | 434.0 |
| 4 | 486.1 |
| 3 | 656.3 |
Paschen series (nl=3)
The series was first observed during the years 1908, by a German physicist Friedrich Paschen. Thus the series is named after him. Paschen series is displayed when electron transition takes place from higher energy states(nh=4,5,6,7,8,…) to nl=3 energy state. All the wavelength of Paschen series falls in the Infrared region of the electromagnetic spectrum. The shortest wavelength of next series, i.e., Brackett series overlap with Paschen series. From this series, all subsequent series overlap.
Refer to the table below for various wavelengths associated with spectral lines.
| Energy level (n) | Wavelength (? in nm) in air |
| ∞ | 820.4 |
| 8 | 954.6 |
| 7 | 1005 |
| 6 | 1094 |
| 5 | 1282 |
| 4 | 1875 |
Brackett series (nl=4)
The series was first observed during the years 1922, by an American physicist Friedrich Sumner Brackett. Thus the series is named after him. Brackett series is displayed when electron transition takes place from higher energy states(nh=5,6,7,8,9…) to nl=4 energy state. All the wavelength of Brackett series falls in Infrared region of the electromagnetic spectrum.
Refer to the table below for various wavelengths associated with spectral lines.
| Energy level (n) | Wavelength (? in nm) in air |
| ∞ | 1458 |
| 9 | 1817 |
| 8 | 1944 |
| 7 | 2166 |
| 6 | 2625 |
| 5 | 4051 |
Pfund series (nl=5)
The series was first observed during the years 1924, by August Harman Pfund. Thus, the series is named after him. Pfund series is displayed when electron transition takes place from higher energy states(nh=6,7,8,9,10,…) to nl=5 energy state. All the wavelength of Pfund series falls in Infrared region of the electromagnetic spectrum.
Refer to the table below for various wavelengths associated with spectral lines.
| Energy level (n) | Wavelength (? in nm) in vacuum |
| ∞ | 2279 |
| 10 | 3039 |
| 9 | 3297 |
| 8 | 3741 |
| 7 | 4654 |
| 6 | 7460 |
Humphreys series (nl=6)
The series was first observed during the years 1953, by an American Physicist Curtis J Humphreys, thus the series is named after him. Humphreys series is displayed when electron transition takes place from higher energy statesnh=7,8,9,10,11…) to nl=6 energy state. All the wavelength of Humphreys series falls in Infrared region of the electromagnetic spectrum.
Refer to the table below for various wavelengths associated with spectral lines.
| Energy level (n) | Wavelength (? in μm) in vacuum |
| ∞ | 3.282 |
| 11 | 4.673 |
| 10 | 5.129 |
| 9 | 5.908 |
| 8 | 7.503 |
| 7 | 12.37 |
Further
They are the unnamed series, which follow the spectral pattern described by the Rydberg equation. They are first observed in infrared range during an experiment in 1972 by Peter Hanson and John Strong. These series are displayed when electron transition takes place from higher energy states(nh=8,9,10,11…) to nl=7 or above energy state. The series is observed in higher wavelength. The spectral lines are extremely faint and widely spread out. They correspond to highly rare atomic events.
Related Physics Links:
| Spectroscopy |
| X-Ray |
| Wavelength of light |
Stay tuned with BYJU’S for latest Physics-related topics.




Comments