Chemistry is a branch of science, studies the substances with which materials are made up of. The best way to learn the subject is to first understand the concepts covered in the subject, as per the West Bengal Board Class 12 Chemistry Syllabus. Everything in and around us composed of chemicals. How they react with one another, their chemical properties, ratios behind mixing of one or more elements to form new etc are well explained under Chemistry. To know the topics and concepts taught in Class 11 Chemistry for the academic year, students can refer to the WBCHSE Class 11 Chemistry Syllabus.
West Bengal state board Class 12 Chemistry Syllabus is prescribed by the West Bengal Council of Higher Secondary Education, popularly known as WBCHSE. The board was established and attained autonomous status in the year 1975 and endeavors for promoting and developing standard affordable higher secondary education in the state territory of West Bengal.
WBCHSE Class 12 Chemistry Syllabus comprises of all three disciplines that is organic chemistry, inorganic chemistry, physical chemistry. The major topics studied include – solid states, solutions, electrochemistry, chemical kinetics, surface chemistry, General principles and processes of isolation of elements, p-block, d-block, f-block elements, coordination compounds,haloalkanes and haloarenes, alcohol, phenol and ethers, aldehydes, ketones and carboxylic acids, organic compounds containing nitrogen, biomolecules, polymers, chemistry in everyday life.
Download WBCHSE Class 12 Chemistry Syllabus 2021-22
Unitwise Marks Distribution From 2021-22 Syllabus
| Unit Name | Marks |
| Solid State* | 04 |
| Solutions* | 06 |
| Electrochemistry* | 05 |
| Chemical Kinetics* | 10 |
| Surface Chemistry* | 07 |
| p-Block Elements* | 08 |
| d-and f- Block Elements* | 01 |
| Coordination Compounds* | 04 |
| Haloalkanes and Haloarenes* | 04 |
| Alcohols, Phenols and Ethers* | 04 |
| Aldehydes, Ketones and Carboxylic acids | 10 |
| Organic Compounds containing Nitrogen* | 04 |
| Bio molecules* | 03 |
| Total | 70 |
The complete latest syllabus of WBCHSE of Class 12 Chemistry is mentioned below along with the subtopics.
Solid State:
Classification of Solids based on different binding forces; molecular, ionic, covalent and metallic solids, amorphous and crystalline solids (elementary idea), unit cell in two-dimensional and three-dimensional lattices, packing efficiency, calculation of density of unit Cell, Packing in solids, voids, number of atoms per unit cell in a cubic unit cell , point defects.
Solutions:
Types of Solutions, expression of concentration of solution of solids in liquids, solubility of gases in liquids, solid solutions, Raoult’s law, colligative properties – relative lowering of vapour pressure, elevation of boiling point, depressing of freezing point, osmotic pressure, determination of molecular masses using colligative properties.
Electrochemistry:
Radox reactions, EMF of cell, standard and electrode potential, Nernst equation and its application to chemical cells, Relation between gibbs energy change and EMF of a Cell, Conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch’s law, electrolysis and laws of electrolysis (elementary idea).
Chemical Kinetics:
Rate of reaction (average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst ‘ order and molecularity of a reaction ; rate law and speci fie rate constant, integrated rate equations and halflife (only for zero and first order reaction)
Surface Chemistry:
Adsorption – Physisorption and chemisorptions, factors affecting adsorption of gases on solids colloidal state, distinction between true solutions, multi molecular and macromolecular colloids; properties of colloids, tyndall effect . Brownian movemen(flectro phoresis, coagulation.
P- Block Elements:
Group 15 elements: General introduction, electronic configuration, occurrence, oxidation states , trends in physical and chemical properties; Nitrogen;– Preparation, properties and uses; compounds of nitrogen’ Preparation and properties of ammonia and nitric acid.
Group 16 elements: General introduction, electronic configuration, Oxidation states, Occurrence, trends in physical and chemical properties, dioxygen, preparation , properties and uses ; classification of oxides, Ozone, sulphur – allotropic forms : compounds of sulpher : preparation , properties and uses of sulphur dioxide, sulphuric acid ; properties and uses : oxiacids of sulphur (structures only).
Group 17 elements: General introduction, electronic configuration, oxidation states , occurrence, trends in physical and chemical properties : compounds of halogens ; Preparation , properties and uses of chlorine and hydrochloric acid, interhalogen compounds, oxiacides of halogens (strncture only).
Group 18 elements: General introduction, electronic configuration, occurance, trends in physical and chemical properties, uses.
d- and f-Block Elements:
General introduction, electronic configuration, occurrence and characteristic of transition metals , general trends in properties of the first now transition metals – metallic character, ionization enthalpy, oxidation states, ionic radi colour, catalytic property, magnetic properties, interstitial compounds, alloy formation. Lanthanoids – electronic configuration, oxidation states and lanthanoid contraction and its consequences.
Coordination Compounds:
Coordination compounds – introduction , ligands, coordination number, colour, magnetic properties and shape, IUPAC nomenclature of mononuclear coordination compounds, bonding, Werner’s theory, VBT and CFT.
Haloalkanes and Haloarens:
Nomenclature, nature of C-X bond, physical and chemical properties, mechanism of substitution reactions, stability of carbonations R-S and d-1 configurations.
Haloarenes: Natue of C-X bond , substitution reactions (directive influence of halogen for monosubstituted compounds only)
Alcohols, Phenols and Ethers:
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols; mechanism of dehydration
Phenols: Nomenclature, methods of Preparation, physical and chemical properties , acidic nature of phenol, electrophillic substitution reactions, uses of phenol.
Ethers: Nomenclature, methods of preparation, Physical and chemical properties uses.
Aldehydes, Ketones and Carboxylic Acids:
Aldehydes and Ketones: Nomenclature , nature of carbonyl group, methods of preparation, physical and chemical properties,. mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldel1ydes uses.
Carboxylic Acids: Nomenclature, acidic nature methods of preparation, physical and chemical properties uses.
Organic Compounds Containing Nitrogen
Nitro compounds: General methods of preparation and chemical reactions.
Amines: Nomenclature, classification, structure methods of preparation, physical and chemical properties, uses, identification of primary secondary and tertiary amines.
Cyanides and isocyanides – will be mentioned at relevant places in context.
Bio Molecules:
Carbohydrates – Classification (Addoses and ketose), monosaccharide’s (glucose and fructose) 0-L configuration
Proteins – Elementary idea of alpha amino acids , peptide bond, polypeptides, proteins, structure of proteins -Primary secondary , tertiary and quaternary structures (qualitative idea only), denaturation of proteins.
Nucleic Acids: DNA and RNA
Few chapters were deleted from the syllabus, the previous year. Details are as mentioned below:
Download WBCHSE Class 12 Chemistry Deleted Portion 2020-21
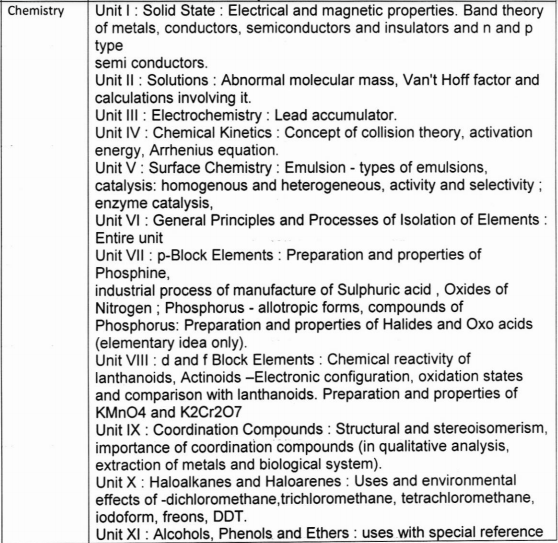

Class 12 Chemistry Practical syllabus
| Evaluation Scheme for Examination | Marks |
| Volumetric analysis | 10 |
| Salt Analysis | 08 |
| Content Based Experiment | 06 |
| Class Record, Viva and Project work | 06 |
| Total | 30 |
Surface Chemistry.
a) Preparation one lyophilic and one lyophobic sol. Lyophilic sol – starch albumin and gum, Lyophobic sol – aluminium hydroxide, ferric hydroxide, arsenious sulphide.
b) Study of the role of emulsifying agents in stabilizing the emulsions of different oils.
Chemical kinetics
a) Effect of concentration and temperature on the rate of reaction between sodium thiosulphate and hydrochloric acid.
b) Study of reaction rates of any one of the following:
Reaction of iodide ion with hydrogen peroxide at room temperature using different concentrations of iodide ions.
Reaction between potassium iodide, KIO3 and sodium sulphate: (Na2So3) using starch solution as indicator (clock reaction).
Thermo chemistry
Any of the following experiments
- Enthalpy of dissolution copper sulphate or potassium nitrate.
- Enthalpy of neutralization of strong acid (HCl) and strong base (NaOH)
- Determination of enthalpy change during interaction (hydrogen bond formation) between acetone and chloroform
Electro chemistry
Variation of cell potential in Zn/Zn2+//Cu2+/Cu with change in concentration of electrolytes (CuSO4 or ZnSO4) at room temperature.
Chromatography
- Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf Values.
- Separation of constituents present in an inorganic mixture containing two cations only (constituents having large difference in Rf values to be provided).
Preparation of Inorganic Components
- Preparation of double salt of ferrous ammonium sulphate or potash alum
- Preparation of potassium ferric oxalate
Preparation of Organic compounds
Preparation of any two of the following compounds
- Acetanilide
- Di benzal acetone
- p-Nitroacetanilide
- Aniline yellow or 2- Napthal aniline dye.
- Iodoform
Tests for the functional groups present in organic compounds:
Unsaturation, alcoholic, phenolic, aldehydic, ketonic, carboxylic and amino (primary) groups.
Characteristic test of carbohydrates, fats and proteins in pure samples and their detection in given food stuffs.
Determination of concentration/ minority of KMnO4 solution by titrating it against a standard solution of:
- Oxalic acid
- Ferrous ammonium sulphate (students will be requied to prepare standard solution by weighing themselves)
Qualitative analysis
Determination of one cation and one anion in a given salt.
Cations – Pb2+, Cu2+, As3+, Al3+, Fe3+, Mn2+, Ni2+, Zn2+, Co2+, Ca2+, Sr2+, Ba2+, Mg2+, NH4+
Anions – CO32-, S2-, SO32-,NO2-–,Cl–, Br–,l-, PO43- ,C2O42-, CH3COO-
(Note; Insoluble salts excluded)
Project work- where feasible may include
- Model preparation
- Investigatory project
- Science exhibits
- Participation in science fairs
- Testing of purity of food articles like butter, pulse and milk etc.
Stay tuned with Byju’s for latest updates on Mathematics, Physics, Chemistry, Biology Textbooks and Previous year question papers of WBCHSE class 12.


Comments