While learning the subject Chemistry, one would certainly come across words like acids and bases in particular.
Table of Contents
Definitions
What is an Acid?
Acids may be defined as the compounds that donate an ion of hydrogen (H+) to another compound (usually called a base). Conventionally, an acid used to be known as the chemical compound that once dissolved in water, produces a solution that has a low activity of hydrogen ion than water in its purest form.
What is a Base?
A base on the other hand which is soluble in nature is termed as an alkali. Liquids that are volatile (acids) once mixed with certain substances would produce salts. The produced salts would form a base that is concrete and thus they were termed as bases. Acids are usually H+ donors while Bases are H+ acceptors.
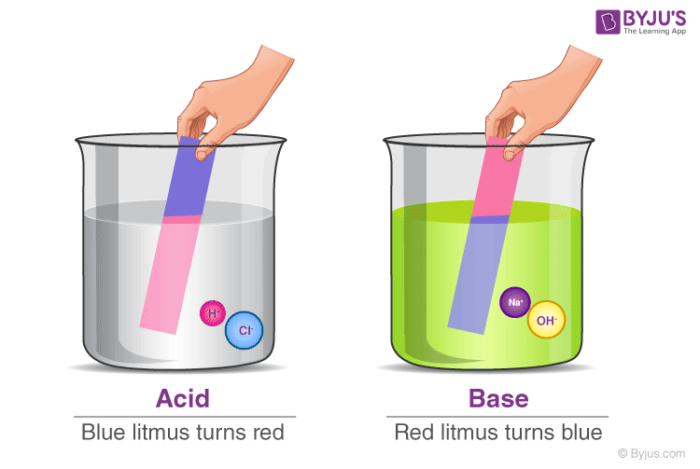
Acid vs Base
To give you a more clear understanding of the differences between acids and bases here is a comparison chart that you can refer to.
| Basis | Acid | Base |
| Definition | An acid is any chemical compound once dissolved in water produces a solution with hydrogen ion activity more than purified water | A base is an aqueous substance that could absorb hydrogen ions. |
| Strength | Relies on the concentration of the hydronium ions | Relies on the concentration of the hydroxide ions |
| Examples | Acetic acid CH3COOH and sulphuric acid | Sodium Hydroxide (NaOH) and Ammonia |
| Characteristics (Physical) | Would depend upon the temperature acids would look solid, liquid or in the form of gas. It would also have a sour taste. | Bases would feel slippery and solid in nature (except for ammonia, which is gaseous). It would have a bitter taste. |
| Disassociation | Acids would release hydrogen ions (h+) when mixed with water | Bases would release hydroxide ions(OH-) when mixed with water |
| Test with Litmus | Would turn litmus paper red | Would turn litmus paper blue |
Recommended Videos
Frequently Asked Questions – FAQs
What is an acid in chemistry?
Any substance that tastes sour in water, turns blue litmus paper red, reacts with some metals to liberate hydrogen, combines with bases to form salts, and encourages chemical reactions is classified as an acid.
What is an acid easy definition?
An acid is a chemical substance that contains hydrogen and can combine with other chemicals to generate salts. It is usually a liquid. When acids come into touch with other things, they can burn or dissolve them.
What is the taste of base?
Bases have a harsh flavour and are less commonly found in foods than acids. Many bases, such as soaps, are slick to touch. Indicators’ colours are also changed by bases.
Is Slippery acid or a base?
The bases have a slick texture. Bases are found in many soaps and detergents. The bases in your shampoo are responsible for the shampoo’s slick texture. Base Reactions (Base Reactions) – Bases, unlike acids, do not react with metals.
What is the pH meaning?
The pH of water is a measurement of how acidic or basic it is. The range is 0 to 14, with 7 being the neutral value. Acidity is indicated by a pH less than 7, while a pH greater than 7 indicates a base. pH is a measurement of the proportion of free hydrogen and hydroxyl ions in water.
The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over. To know more about acids, bases and other interesting topics on the subject you can keep visiting BYJU’S or download the app.


Comments