What is Clemmensen Reduction?
The Clemmensen reduction is a reaction that is used to reduce aldehydes or ketones to alkanes using hydrochloric acid and zinc amalgam. The Clemmensen reduction is named after a Danish chemist, Erik Christian Clemmensen.
Clemmensen Reduction Reaction
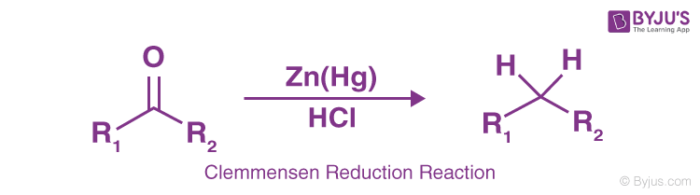
This reaction is particularly effective in aryl-alkyl ketones reduction formed in Friedel-Crafts acylation. The reaction is more effective in reduction of cyclic ketones or aliphatic and zinc metal.
Examples
Example 1

Clemmensen reduction example 1
Example 2

Clemmensen reduction example 2
Example 1

Clemmensen reduction example 3
Mechanism of Clemmensen Reduction Reaction:
The mechanism of this reaction is not completely understood, but there are two proposals;
-
Carbanionic mechanism:
The carbanionic mechanism of reaction shows that the zinc attacks directly to the protonated carbon.
-
Carbenoid mechanism:
While the carbenoid mechanism is a radical process and reduces the happenings on zinc metal surface. The reduction takes place at the surface of the zinc catalyst. In this reaction, alcohols are not postulated as intermediates, because subjection of the corresponding alcohols to these same reaction conditions does not lead to alkanes.
The following proposal employs the intermediacy of zinc carbenoids to rationalize the mechanism of the Clemmensen Reduction:
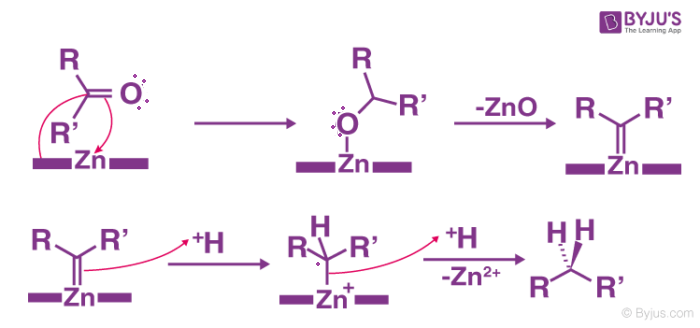
The underlying substance must not react to the acidic conditions. The acid sensitive base substance reacts in the Wolff-Kishner reduction that has a strong base if it is milder than it is Mozingo reduction. The reaction is not for the substances that are sensitive to acids.
The heterogeneous nature makes the mechanism remains obscure, in spite of its antiquity and the studies on mechanism are difficult. There are only a few studies on the particular reaction proposed like possibly zinc carbenoids and organozinc intermediates.
To learn more about other Important Chemical Equations For Chemistry Class 12, register with BYJU’S and download our app.


Comments