What is Dehydration of Alcohols?
Alcohol upon reaction with protic acids tends to lose a molecule of water to form alkenes. These reactions are known as dehydrogenation or dehydration of alcohols.
It is an example of an elimination reaction. Its rate varies for primary, secondary and tertiary alcohols. This variation of rate can be attributed to the stability of carbocation generated. Since the carbocation is most stable in the case of tertiary alcohols, the rate of dehydration is highest for tertiary alcohols in comparison to secondary and primary alcohols.
Dehydrogenation is the removal of hydrogen from the feedstock, such as the treatment of paraffin for the production of olefin. The degree of dehydrogenation during thermal cracking of petroleum varies with the starting material and operating conditions, but due to its practical significance, methods have been found to increase the level of dehydrogenation and, in some cases, to make it almost the only reaction.
Dehydrogenation
Dehydrogenation is one of the most important processes in the chemistry of petroleum because it turns the starting inert alkanes into olefins and aromatic compounds, starting points towards other functional groups.
Catalytic dehydrogenation plays an significant role in the development of olefin light (C3–C4 carbon range), detergent range (C10–C13 carbon range), and dehydrogenation to styrene by ethylbenzene. During the Second World War, catalytic dehydrogenation of butane over a catalyst for chromium – alumina was practiced to generate butenes that were dimerized to octenes and hydrogenated to octans to yield high octane aviation fuels.
Dehydrogenation is a highly endothermic process, and as such, a restricted reaction to the equilibrium. While important aspects of dehydrogenation include reaching equilibrium or conversion to near-equilibrium while reducing side reactions and coke formation.
Recommended Videos

Dehydrogenation Reaction
Dehydration Mechanism Steps
If either Raney-Ni, Al(i-ORr)3, or alumina are absent from the catalytic mixture, secondary alcohol dehydrogenation reaction to ketones does not occur. When the Raney-Ni is substituted with other Ni(II) salts I e. NiCl2) or complexes I e. Ni(PPh3)2Cl2), no reaction is noted.
Dehydration of alcohols follows a three-step mechanism.
- Formation of protonated alcohol
- Formation of carbocation
- Formation of alkenes
General dehydration reaction of alcohols can be seen as,
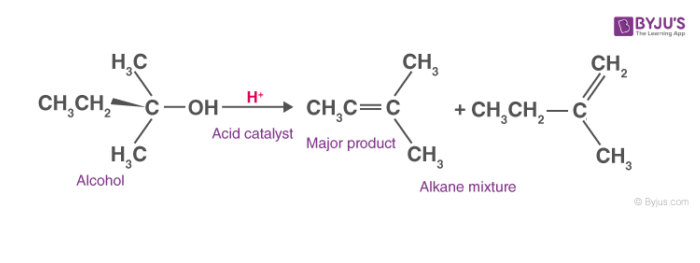
Mechanism of Dehydration of Alcohols:
Dehydration of alcohols can follow E1 or E2 mechanism. For primary alcohols, the elimination reaction follows E2 mechanism while for secondary and tertiary alcohol elimination reaction follows E1 mechanism.
Generally, it follows a three-step mechanism. The steps involved are explained below.
1. Formation of protonated alcohol:
In this step, the alcohol is acted upon by a protic acid. Due to the lone pairs present on the oxygen atom it acts as a Lewis base. Protonation of alcoholic oxygen takes place which makes it a better leaving group. It is a reversible step which takes place very quickly.

2. Carbocation formation:
In this step, the C-O bond breaks generating a carbocation. This step is the slowest step in the mechanism of dehydration of an alcohol. Hence, the formation of the carbocation is considered as the rate-determining step.
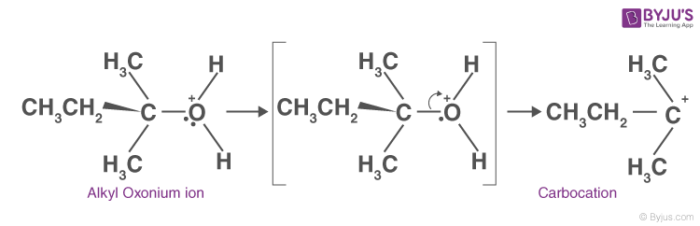
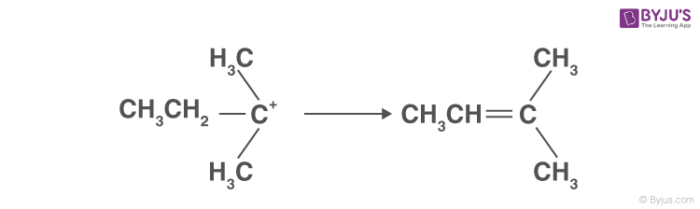
3. Alkene formation:
This is the last step in the dehydration of alcohols. Here the proton generated is eliminated with the help of a base. The carbon atom adjacent to the carbocation breaks the existing C-H bond to form C=C. Thus, an alkene is formed.
Dehydrogenation and rehydrogenation reactions are reversible and the reagents and components are recyclable as a result of which the device can be used more efficiently as a hydrogen supply network technology compared to other hydrogen storage materials.
For a detailed discussion on dehydration of alcohols, please download BYJU’S – The Learning App.


Comments