What are Reducing Agents?
A substance which loses electrons to other substances in a redox reaction and gets oxidized to the higher valency state is called a reducing agent.
A reducing agent is one of the reactants of an oxidation-reduction reaction which reduces the other reactant by giving out electrons to the reactant. If the reducing agent does not pass electrons to other substance in a reaction, then the reduction process cannot occur.

Recommended Videos

Characteristics of Reducing Agent
- Reducing agents tend to give away electrons. The metals of the s-block in the periodic table are said to be good reducing agents.
- The reducing agent after losing electrons gets oxidized and also causes the opposite reactant to get reduced by supplying electrons.
- All the good reducing agents have the atoms which have low electronegativity and a good ability of an atom or a molecule to attract the bonding electrons and the species having very small ionization energies.
- All the oxidation and reduction reactions involve the transfer of electrons.
- When some substance is oxidized, it is said to lose electrons and the substance which receives electrons is said to be reduced.
- If the substance has a strong tendency to lose electrons, then it is said to be a strong reducing agent (since it will reduce the other substances by donating electrons).
Strong Vs Weak Reducing Agent
The stronger the reducing agent, the weaker is the corresponding oxidizing agent. Fluorine gas is known to be a strong oxidizing agent and whereas F- is said to be a weak reducing agent. We also know that – the weaker an acid then stronger is the conjugate base. Similarly, the weaker the oxidizing agent than the more strong is the corresponding reducing agent as shown in the figure below.
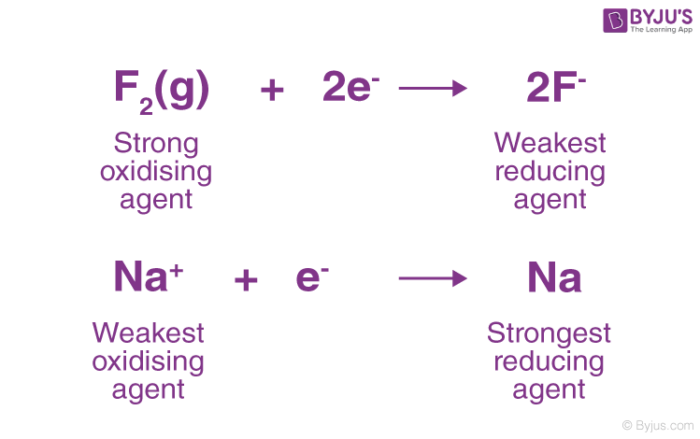
Reducing Agent Example
Some common reducing agents include metals such as Na, Fe, Zn, Al and non-metals such as C, S, H2. Some compounds and also the Hydracids such as HCl, HI, HBr, H2S behave as good reducing agents. A brief explanation over some reducing agents are given below-
- Lithium– Lithium is a chemical element with atomic number 3 and a symbol Li. It appears like a soft and silvery-white metal and belongs to the alkali metal group of the periodic table. It is said to be a strong reducing agent when placed in solutions.
- Iodides– The salts of Iodides are said to be mild reducing agents. They react with oxygen to give out iodine. These also possess various antioxidant properties.
- Reducing sugars– Reducing sugars are those which behave in a similar as that of the reducing agents because of the free ketone group or a free aldehyde group present. All monosaccharides along with disaccharides, polysaccharides, oligosaccharides are said to be reducing sugars.
Frequently Asked Questions – FAQs
What is the strongest reducing agent?
Due to the smallest standard reduction potential, lithium is the strongest reduction agent. It decreases another substance when something is oxidized, becoming a reduction agent. Lithium is, therefore, the most powerful reducing agent.
What is the weakest reducing agent?
The highest oxidizing agent is the weakest reducing agent. The species that are lowered in a redox reaction are oxidizing agents.
Why hydrogen is a good reducing agent?
When hydrogen gas is carried over warm metallic oxides of copper, lead, iron, etc., it removes oxygen from them and lowers them to their respective metal.
Is Iodine a reducing agent?
Iodide ions are more effective reduction agents than bromide ions. The focused sulphuric acid oxidizes them to iodine.
What Colour is iodine in the water?
The complex formation changes the colour of the absorbed light. In water, an iodine solution is yellow-brown rather than violet.
Some other compounds of reducing agents include Carbon, Carbon monoxide, Ascorbic acid, Sulphur dioxide, Hydrogen, Oxalic acid, Phosphites, phosphorous acid, hypophosphites, etc.


Comments