What is a Saturated Solution?
A saturated solution is a solution that contains the maximum amount of solute that can be dissolved under the condition at which the solution exists.
In chemistry, after studying solutions and properties of the solution, one can understand that a solution can reach a status of saturation. This state is when the solution has reached a point in which no more solute can be added. Addition of solute after this point would result in a solid precipitate or gas being released. Such a mixture is called a saturated solution.
How to Prepare a Saturated Solution?
A saturated solution is prepared by continuously adding solute to the solution until a stage is reached where the solute appears as a solid precipitate or as crystals to form a highly saturated solution.
- Consider the process of adding table sugar to a container of water.
- Initially, the added sugar dissolves as the solution is stirred.
- Finally, as more sugar has added a point is reached where no amount of stirring will cause the added sugar to dissolve.
- The last added sugar remains as a solid on the bottom of the container, the solution is saturated.
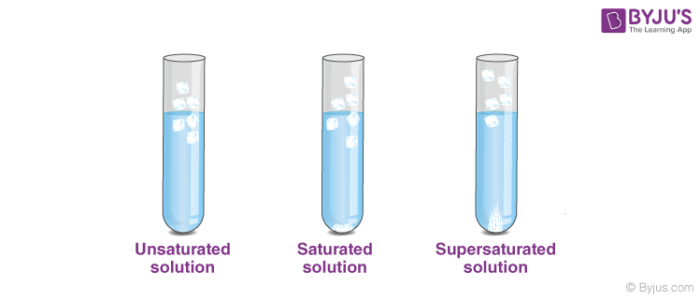
Types of Saturation
The main three types of saturation are explained below.
| Types of Saturation | Definitions | Example |
| Saturated Solution | A saturated solution is a solution that is in equilibrium with respect to a given dissolved substance. | Carbonated water |
| Unsaturated Solution | A solution not in equilibrium with respect to a given dissolved substance and in which more substance can be dissolved. | NaCl in water |
| Supersaturated Solution | A solution contains more dissolved substance than a saturated solution does. | Sugar is dissolved in saturated solution by heating |
To Know About Solvents And Solutes, Watch The Below Video:

Factors affecting the Point of Saturation
- With an increase in temperature the solubility of ionic solutions increases, except mixtures which are made up of compounds containing anions.
- Solutes which are finely divided possess greater solubility.
- The rate of crystallization is dependent upon the amount of solute at the crystal surface.
- The solution is said to be saturated if the rate of crystallization and the rate of solubility are the same.
- The net dissolving rate can be increased by stirring the solution which prevents the build-up of solute.
- The response of the equilibrium system is predicted using Le Chatelier’s principle which depends upon the change in pressure, concentration or temperature.
Everyday Examples of Saturated Solution
- Beverages are one of the most widely used and loved saturated solutions. In these drinks, water is a solvent and carbon is bombarded as a solute until the point of saturation is reached.
- In the kitchen, many cooking recipes involves dissolving of salt, sugar and other household ingredients into the water. This procedure is temperature-dependent. As the temperature of water increases the solubility of the solute increases. After the point of saturation is reached the solute forms a visible layer on top of the solvent.
- Soil present on the earth surface can also be called as a saturated mixture which consists of nitrogen. Once the saturation point is reached; the excess nitrogen is let out into the air in the form of gas.
Join BYJU’S to clarify your doubts by the expert mentors.


Comments