What are Hydrocarbons?
Hydrocarbons are organic compounds that are entirely made up of only two kinds of atoms – carbon and hydrogen. Typically, hydrocarbons are colourless gases that have very weak odours. Hydrocarbons can feature simple or relatively complex structures and can be generally classified into four subcategories, namely alkanes, alkenes, alkynes, and aromatic hydrocarbons. The study of hydrocarbons can provide insight into the chemical properties of other functional groups and their preparation. Furthermore, hydrocarbons such as propane and butane are used for commercial fuel purposes in the form of Liquefied Petroleum Gas (LPG). Benzene, one of the simplest aromatic hydrocarbons, serves as the raw material for the synthesis of many synthetic drugs.
Download hydrocarbons JEE Previous Year Solved Questions PDF

Structure of Hydrocarbon
Table of Content
The molecular formula for these compounds is CxHy. The existence of hydrocarbons is seen in plants and trees. For example, Carotenes is an organic pigment that is found in green leaves and carrots. These hydrocarbons make up to 98% of natural crude rubber. Further, they possess large internal energy which renders them their importance.
⇒ Check: Basic Concepts of Organic Chemistry
Classification and Types of Hydrocarbons
Older chemists classified hydrocarbons as either aliphatic or aromatic. The classification was done based on their source and properties. As such, it was found that Aliphatic hydrocarbons were derived from the chemical degradation of fats or oils whereas aromatic hydrocarbons contained substances that were a result of the chemical degradation of certain plant extracts. However, today we classify hydrocarbons on the basis of structure and not merely on the origin.
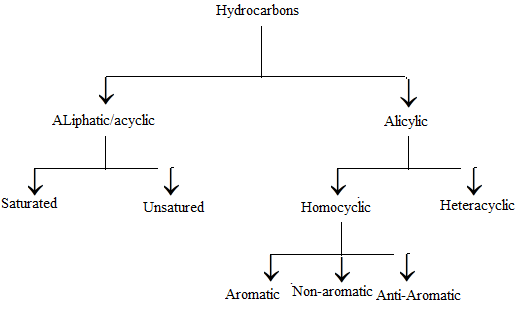
Classification of Hydrocarbons
Types of Hydrocarbons
- Saturated Hydrocarbons: In these compounds, carbon-carbon atoms and carbon-hydrogen atoms are held together by single bonds. These single bonded compounds are the simplest hydrocarbons. These types of hydrocarbons don’t have double or triple bonds. In terms of hybridization, they have Sp3 hybridised carbon atom with no Sp2 or Sp hybridised carbon atoms. They are together called alkanes which have a general formula CnH2n+2. For example, CH4C3H6.
- Unsaturated Hydrocarbons: These compounds consist of a single, double or a triple bond between carbon-carbon atoms. The double-bonded compounds are called alkenes and the triple bonded compounds are called alkynes. The general formula for alkenes is CnH2n and for alkynes the general formula is CnH2n-2.
- Cycloalkanes: These hydrocarbons possess one or multiple carbon rings. The hydrogen atom is attached to the carbon ring.
- Aromatic Hydrocarbons: These are also called arenes. Arenes are compounds which consist of at least one aromatic ring.
- Aliphatic Hydrocarbons: They are straight chain structures having no rings in them.
- Alicyclic Hydrocarbons: They are hydrocarbons having a ring structure in them. The carbons atoms can be Sp, Sp2 or Sp3 hybridised.
Properties of Hydrocarbons
Due to their different molecular structures, the empirical formula of hydrocarbons is also different from each other. For instance, alkanes, alkynes or alkenes, the amount of bonded hydrogen decreases in alkenes and alkynes. This is mainly due to the “self-bonding” or catenation of carbon that prevents the complete saturation of the hydrocarbon by the formation of double or triple bonds. The ability of hydrocarbons to bond to themselves is known as catenation. With such capabilities, they can form more complex molecules like cyclohexane and in rare instances, aromatic hydrocarbons like benzene.
Meanwhile, cracking of Hydrocarbons is a process in which heavy organic molecules are broken down into lighter molecules. This is accomplished by supplying an adequate amount of heat and pressure. Sometimes catalysts are used to speed up the reaction. This process plays a very important role in the commercial production of diesel fuel and gasoline.
⇒ Check: Combustion of Hydrocarbons
Physical Properties
Alkanes with 10 C-atoms or less are generally gases at room temperatures more than 10 C-atoms, the molecules are gases or liquid. Alkanes generally have low boiling and melting points owing to their weak Vanderwal’s interaction.
Boiling point depends on the following factors:
- Molecular mass
- Branching
Alkanes having high molecular mass and high boiling points. Eg: C2H6 has more boiling point than CH4
Alkanes having the same molecular mass but having a different number of branches, the one with less branching has more boiling point this is because of Vanderwal’s force weak as the area increases.
For example, CH3-CH2-CH2-CH3 has more boiling point. Alkanes are very feebly soluble in water but they are soluble in non-polar solvents such as Benzene, CCl4, etc.
Preparation of Hydrocarbons – Alkanes
From alkenes and alkynes
The alkanes can be produced from alkenes or alkynes through hydrogenation. H2 gas is passed over a metal surface such as Ni, Pt along with the alkenes to produce alkane.
CH2=CH2 → (+Hz/Ni) CH3-CH3
The above reaction is called “Sabatier-Sender son’s” reaction. Other catalysts which can be used are Pt, Pd-BaSo4, Adams catalyst (Pt2O) or Wilkinson catalyst (R3PRhCl), etc.
From Alkyl Halides
Alkyl halides can be converted to alkanes through various methods. They are as follows.
1. Using Zn/Protic solvents
2. Using courts reactions
Note: Alkanes with only even number of carbons atoms can be produced.
3. Using Reducing Agents:
R-X → [H]R – H
The reducing agents which can be used are LiAlH4, NaBH4, NaNH2, etc.
Note:
- LiAlH4 can’t reduce 3° halides.
- NaBH4 can’t reduce 1° halide.
2. From Aldehydes/Ketones:
3. From Carboxylic acids through Decarboxylation:
- Kolbe’s Electrolysis
- Using soda-lime
⇒ Also Read: Pyrolysis of Hydrocarbons
Preparation of Hydrocarbons – Alkenes
General formula: CnH2n
Preparation Methods
Most of the reactions involving the preparation of alkenes involve elimination process. There are 3 mechanisms suggested for the elimination reactions. All these eliminations are β- eliminations.
E2 Mechanism
- Second order kinetics.
- Single step process.
- Order & reactivity 1° > 2° > 3°
Because of steric hindrance
- More favoured in non-polar, aprotic solvents.
- Less substituted alkenes formed as a major product.
E1 Mechanism
- Two step process.
- 1st order kinetics
- Order of reactivity: 3° > 2° > 1°
Because of the stability of carbonation.
- More favoured by polar, protic solvents.
- Rearrangement possible
- Gives more substituted alkene as major products.
Hydration
(i) Acid catalysed
- Markovnikov product
- Rearrangement is possible.
(ii) Hydroboration-oxidation:
- Anti – Markonikov
- No rearrangement
(iii) Oxymercuration-Demercuration:
- Markonikov
- No rearrangement
Oxidation Reactions
- Using Baeyer’s reagent
- Using hot KMnO4
- Ozonolysis
- Using O5O4
- Addition of peroxy acid
Preparation of Hydrocarbons – Alkynes
Alkynes can be prepared from alkyl halides and alcohols.
Addition reaction:
All addition reactions in alkenes are possible.
Benzene – Preparation
- From ethyne
- From phenol
- From aniline
Chemical Properties:
Benzene generally undergoes electrophilic substitution reactions.
Uses of Hydrocarbons
- Hydrocarbons are widely used as fuels. For example LPG (liquefied petroleum gas), CNG (Liquefied natural gas).
- They are used in the manufacturing of polymers such as polyethene, polystyrene etc.
- These organic compounds find their application in the manufacturing of drugs and dyes as a starting material.
- They serve as lubricating oil and grease.
Hydrocarbons – Important Topics
Hydrocarbons – Important Questions



That article is very good and simple for understanding the concept of hydro-carbons. it also described the types of hydrocarbons.