Zeroth law of thermodynamics is one of the four laws of thermodynamics. The credit for formulating the law goes to Ralph H. Fowler. Interestingly, the zeroth law of thermodynamics was actually developed much later than the original three laws. However, there was some confusion regarding the nomenclature, whether it should be named the fourth law or some other name. The complication arose because the new law gave a much clearer definition of the temperature and basically replaced what the other three laws had to state. Fowler finally came up with the name to end this conflict.
The zeroth law of thermodynamics frames an idea of temperature as an indicator of thermal equilibrium.
What is Zeroth Law of Thermodynamics?
When a body ‘A’ is in thermal equilibrium with another body ‘b’, and also separately in thermal equilibrium with a body ‘C’, then body ‘B’ and ‘C’ will also be in thermal equilibrium with each other. This statement defines the zeroth law of thermodynamics. The law is based on temperature measurement.
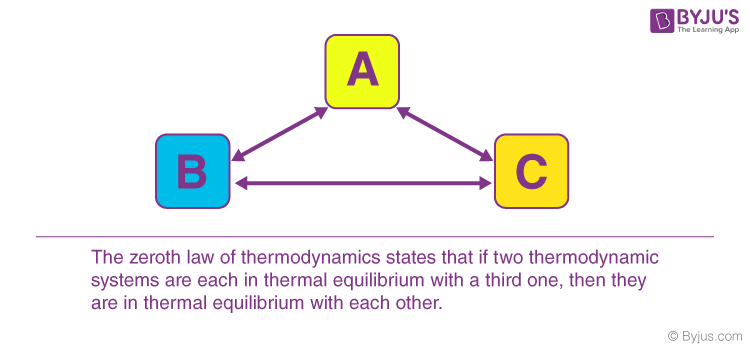
There are also various ways to state the zeroth law of thermodynamics. However, in simple terms, it can be said, “Systems that are in thermal equilibrium exist at the same temperature”.
Zeroth law of thermodynamics takes into account that temperature is something worth measuring because it predicts whether the heat will transfer between objects or not. This is true regardless of how the objects interact. Even if two objects are not in physical contact, heat still can flow between them, by means of radiation mode of heat transfer. Whereas, zeroth law of thermodynamics states that, if the systems are in thermal equilibrium, no heat flow will take place.
Also Read:
| First Law of Thermodynamics |
| Second Law of Thermodynamics |
| Third Law of Thermodynamics |
Thermal Equilibrium
Temperature is a property that distinguishes thermodynamics from other sciences. This property can distinguish between hot and cold. When two or more bodies at different temperatures are brought into contact then after some time they attain a common temperature and they are said to exist in thermal equilibrium.
Systems are said to be in thermal equilibrium if there is no heat transfer, even if they are in a position to transfer heat, based on other factors. For example, if we put food in the refrigerator overnight then that food is in thermal equilibrium with the air of that refrigerator. Heat no longer flows from food to the air or from the air to the food, this state is known as thermal equilibrium.
Zeroth Law of Thermodynamics Example and Applications
The law is important for the mathematical formulation of thermodynamics or more precisely for stating the mathematical definition of temperature. This law is mostly used to compare temperatures of different objects.
If we want to measure the accurate temperature, a reference body is required and a certain characteristic of that body which changes with temperature. The change in that characteristic may be taken as an indication of a change of temperature. That selected characteristic is known as thermodynamic property.
Also Read: Intensive and Extensive Properties of Matter
Nonetheless, the most common application of the zeroth law of thermodynamics can be seen in thermometers. We can observe the zeroth law in action by taking a very common thermometer having mercury in a tube. As the temperature is increased this mercury expands since the area of the tube is constant. Due to this expansion, the height is increased. Now, the increase in the height of the mercury label shows the changes in temperature and basically helps us to measure it.
There are different kinds of thermometers that can be used depending on their thermometric property. They are as follows.
| Thermometer | Thermometric Property |
| Constant volume gas thermometer | Pressure |
| Constant pressure gas thermometer | Volume |
| Electrical resistance thermometer | Resistant |
| Thermocouple | Thermal e.m.f |
| Mercury -in -glass thermometer | Length |
Similarly, another example of the zeroth law of thermodynamics is when you have two glasses of water. One glass will have hot water and the other will contain cold water. Now if we leave them in the table for a few hours they will attain thermal equilibrium with the temperature of the room.


